Drug Impurities Reference Standards
Showing 1051–1060 of 1775 results
-
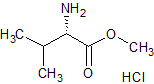
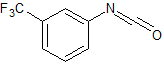
Valsartan ImpurIty 12
- Product Number CT-01110-130
- Parent Drug Valsartan
- CAS Number 6306-52-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
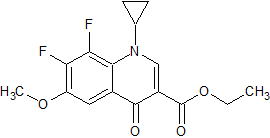
Moxifloxacin Impurity 11
- Product Number CT-01110-182
- Parent Drug Moxifloxacin
- CAS Number 1329836-33-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
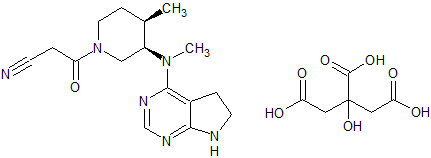
Tofacitinib Impurity 14
- Product Number CT-01110-227
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
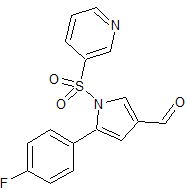
Vonoprazan Fumarate Impurity 42
- Product Number CT-01110-59
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
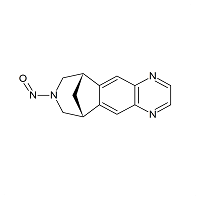
Varenicline N-Nitroso
- Product Number V-10312-01
- Parent Drug Varenicline
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Tadalafil Impurity 12
- Product Number CT-01110-256
- Parent Drug Tadalafil
- CAS Number 171596-44-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Rivaroxaban Impurity 42
- Product Number CT-01110-301
- Parent Drug Rivaroxaban
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
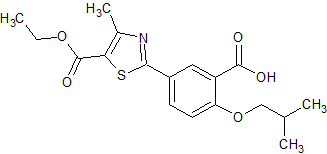
Linagliptin Impurity 31
- Product Number CT-01110-354
- Parent Drug Linagliptin
- CAS Number 666816-98-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Sorafenib Impurity 20
- Product Number CT-01110-411
- Parent Drug Sorafenib
- CAS Number 329-01-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Febuxostat Impurity 58
- Product Number CT-01110-464
- Parent Drug Febuxostat
- CAS Number 2095166-41-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options