Drug Impurities Reference Standards
Showing 1131–1140 of 1775 results
-
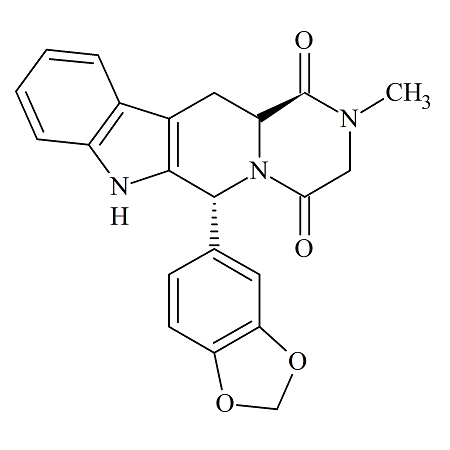
Tadalafil Epimer
- Product Number TAD-08-003
- Parent Drug Tadalafil
- CAS Number 171596-27-3
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
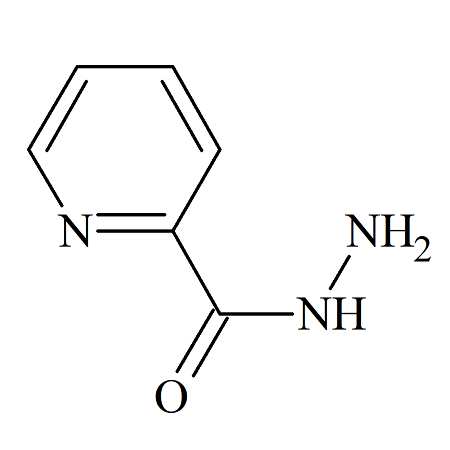
Picolinohydrazide
- Product Number ISZ-16-001
- Parent Drug Isoniazid
- CAS Number 1452-63-7
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 week(s)See more size options -
Budesonide Impurity 1
- Product Number ACA-160910-0008
- Parent Drug Budesonide
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
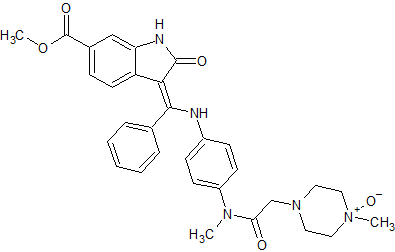
Nintedanib N-Oxide
- Product Number N-01029-01
- Parent Drug Nintedanib
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
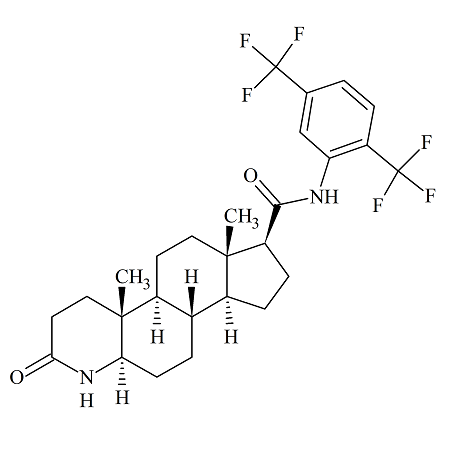
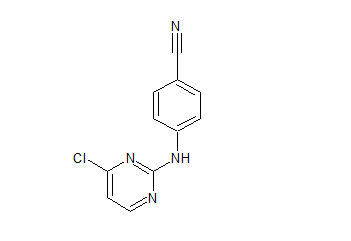
Rilpivirine Chloropyrimidinyl Certified Impurity Reference Standard
- Product Number R-00211-05
- Parent Drug Rilpivirine
- CAS Number 244768-32-9
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
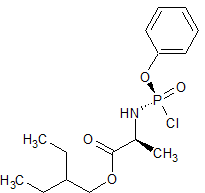
Remdesivir Chloro Phosphate Impurity
- Product Number R-00720-07
- Parent Drug Remdesivir
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Flecainide Imurity B
- Product Number P-30916-01
- Parent Drug Flecainide
- CAS Number 22990-77-8
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
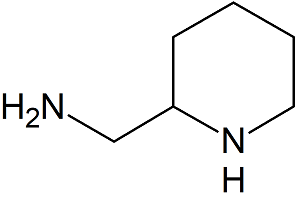
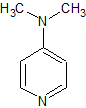
4-Dimethyl Aminopyridine
- Product Number B-71123-0007
- Parent Drug Penciclovir
- CAS Number 1122-58-3
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options