Drug Impurities Reference Standards
Showing 1151–1160 of 1775 results
-
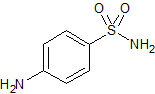
Sulfadimethoxine EP Impurity E
- Product Number CT-01110-858
- Parent Drug Sulfadimethoxine
- CAS Number 63-74-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
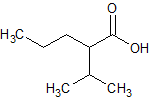
Sodium Valproate EP Impurity C
- Product Number CT-01110-484
- Parent Drug Valproic Acid
- CAS Number 62391-99-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
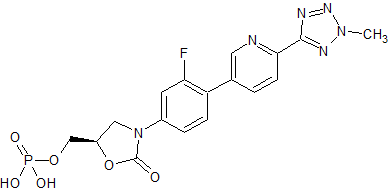
Tedizolid Impurity 36
- Product Number CT-01110-120
- Parent Drug Tedizolid
- CAS Number 1239662-47-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
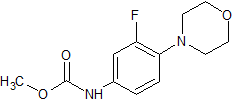
Linezolid Impurity 18
- Product Number CT-01110-167
- Parent Drug Linezolid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
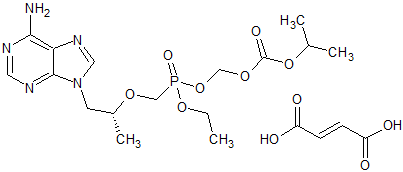
Tenofovir disoproxil Impurity 12
- Product Number CT-01110-211
- Parent Drug Tenofovir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 17
- Product Number CT-01110-48
- Parent Drug Vonoprazan
- CAS Number 1883595-38-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
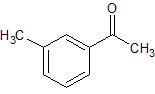
Celecoxib Impurity 20
- Product Number CT-01110-97
- Parent Drug Celecoxib
- CAS Number 585-74-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
(3-9)-Oxytocin
- Product Number CT-01110-1107
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
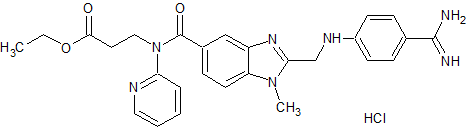
Dabigatran Impurity 25
- Product Number CT-01110-289
- Parent Drug Dabigatran
- CAS Number 211914-50-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
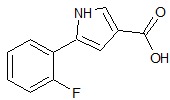
Olmesartan Impurity 30
- Product Number CT-01110-341
- Parent Drug Olmesartan
- CAS Number 157356-73-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options