Drug Impurities Reference Standards
Showing 1341–1350 of 1775 results
-
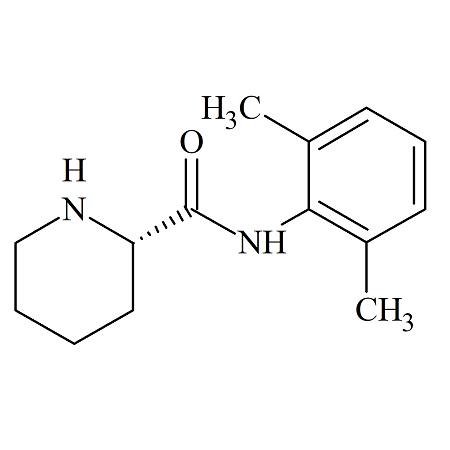
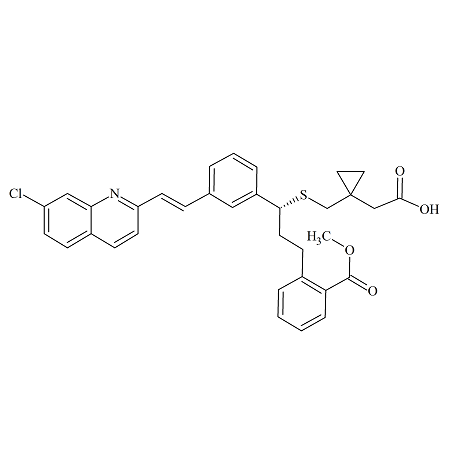
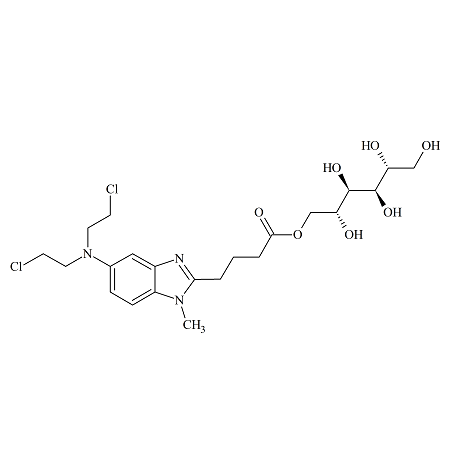
Zafirlukast m-Tolyl Isomer
- Product Number ACB-170824-0006
- Parent Drug Zafirlukast
- CAS Number 1159195-69-3
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
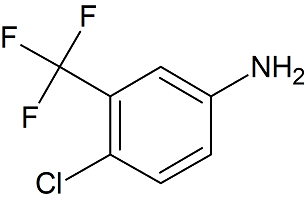
Sorafenib EP Impurity C
- Product Number S-90507-03
- Parent Drug Sorafenib
- CAS Number 320-51-4
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
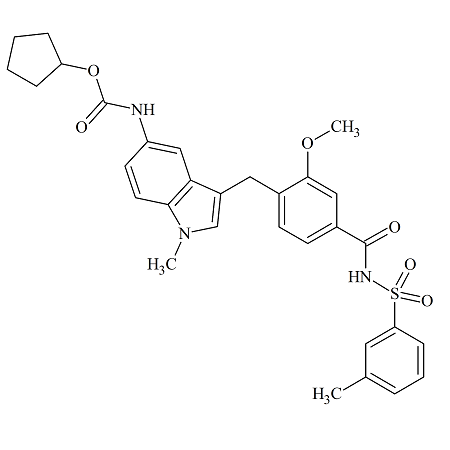
Montelukast EP Impurity H
- Product Number MON-12-002
- Parent Drug Montelukast
- CAS Number 851755-56-1
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
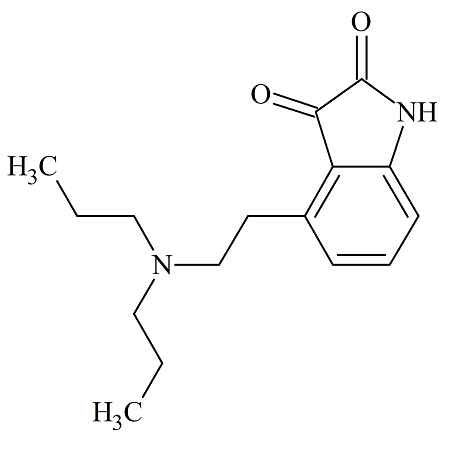
Aprepitant Stage-III (Impurity-D)
- Product Number ACA-160919-0003
- Parent Drug Aprepitant
- CAS Number 155742-64-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
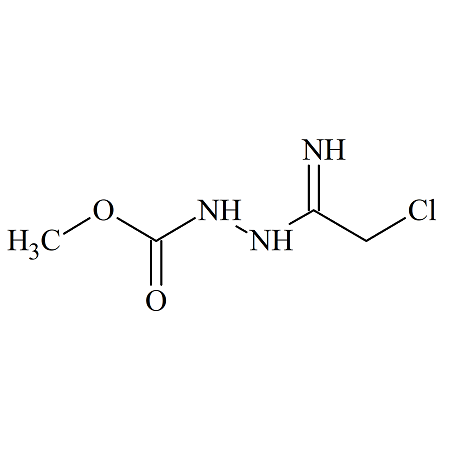
Pramipexole Dihydrochloride Impurity BI-II786BS
- Product Number P-90729-01
- Parent Drug Pramipexole
- CAS Number 1244656-98-1
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
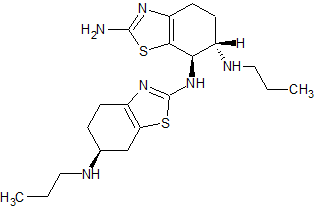
Nilotinib Related Compound A USP Impurity Certified Reference Standard
- Product Number NIL-15-002
- Parent Drug Nilotinib
- CAS Number 641571-11-1
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 week(s)See more size options -
Acyclovir Impurity B
- Product Number ACA-161005-0002
- Parent Drug Acyclovir
- CAS Number 73-40-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options