Drug Impurities Reference Standards
Showing 1371–1380 of 1775 results
-
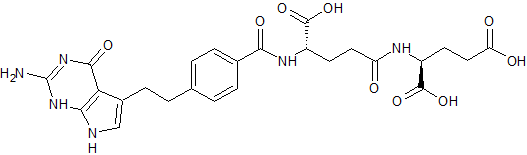
Pemetrexed EP Impurity D
- Product Number CT-01110-966
- Parent Drug Pemetrexed
- CAS Number 144051-68-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
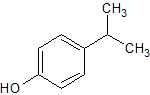
Propofol EP Impurity H
- Product Number CT-01110-830
- Parent Drug Propofol
- CAS Number 99-89-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
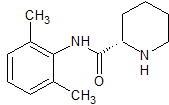
Ropivacaine EP Impurity B
- Product Number CT-01110-970
- Parent Drug Ropivacaine
- CAS Number 27262-40-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
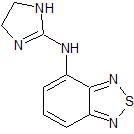
Tizanidine EP Impurity A
- Product Number CT-01110-810
- Parent Drug Tizanidine
- CAS Number 51322-69-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
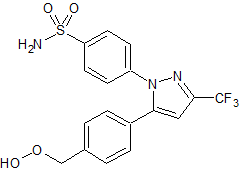
Celecoxib Impurity 23
- Product Number CT-01110-100
- Parent Drug Celecoxib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
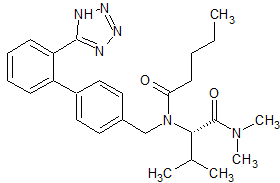
Valsartan Impurity 53
- Product Number CT-01110-140
- Parent Drug Valsartan
- CAS Number 137863-42-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
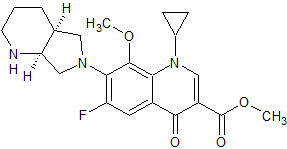
Moxifloxacin Methyl Ester
- Product Number CT-01110-194
- Parent Drug Moxifloxacin
- CAS Number 721970-35-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
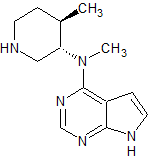
Tofacitinib Impurity 42
- Product Number CT-01110-238
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
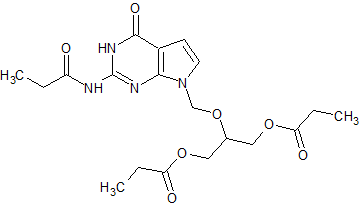
Ganciclovir EP Impurity J
- Product Number CT-01110-71
- Parent Drug Ganciclovir
- CAS Number 177216-32-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
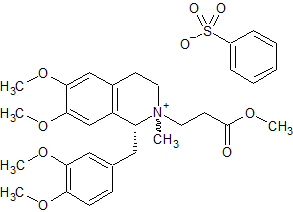
Atracurium Impurity 32
- Product Number CT-01110-1041
- Parent Drug Atracurium
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options