Drug Impurities Reference Standards
Showing 1411–1420 of 1775 results
-
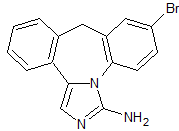
Tavaborole Borolane Impurity Certified Reference Standard
- Product Number B-70915-0007
- Parent Drug Tavaborole
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
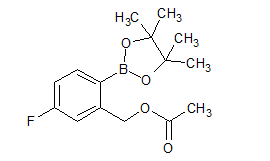
Empagliflozin Impurity 3
- Product Number CT-01110-153
- Parent Drug Empagliflozin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
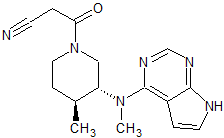
Tofacitinib Impurity 1
- Product Number CT-01110-225
- Parent Drug Tofacitinib
- CAS Number 1092578-46-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
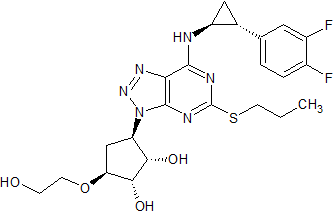
Ticagrelor Impurity 3
- Product Number CT-01110-315
- Parent Drug Ticagrelor
- CAS Number 2096989-56-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
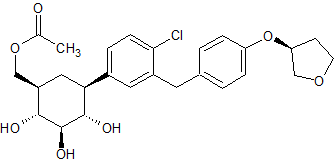
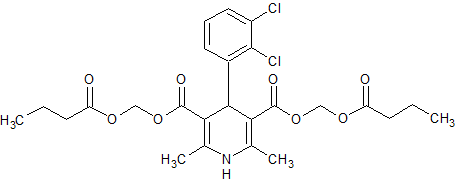
Clevidipine butyrate Impurity 3
- Product Number CT-01110-38
- Parent Drug Clevidipine
- CAS Number 253597-19-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Potassium Clavulanate EP Impurity M
- Product Number CT-01110-450
- Parent Drug Clavulanic acid
- CAS Number 3033-62-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
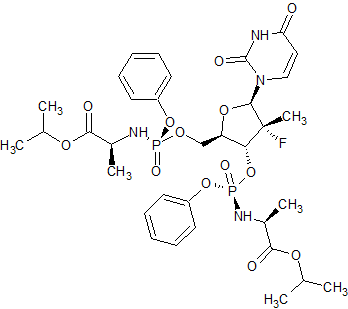
Sofosbuvir Impurity 48
- Product Number CT-01110-106
- Parent Drug Sofosbuvir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
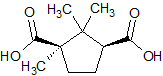
Levocarnitine EP Impurity B
- Product Number CT-01110-617
- Parent Drug Carnitine
- CAS Number 124-83-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
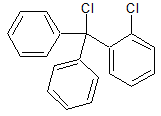
Clotrimazole EP Impurity C
- Product Number CT-01110-870
- Parent Drug Clotrimazole
- CAS Number 42074-68-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Epinastine EP Impurity B
- Product Number CT-01110-1030
- Parent Drug Epinastine
- CAS Number 1217052-16-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options