Drug Impurities Reference Standards
Showing 1431–1440 of 1775 results
-
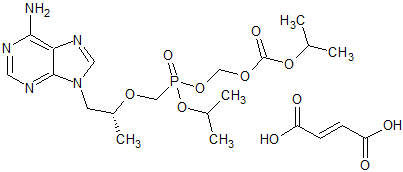
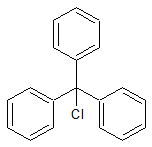
Tenofovir disoproxil Impurity 11
- Product Number CT-01110-210
- Parent Drug Tenofovir
- CAS Number 1422284-15-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Fluconazole Impurity 20
- Product Number CT-01110-46
- Parent Drug Fluconazole
- CAS Number 288-88-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Celecoxib Impurity 19
- Product Number CT-01110-96
- Parent Drug Celecoxib
- CAS Number 577-16-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
(4-9)-Oxytocin
- Product Number CT-01110-1106
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
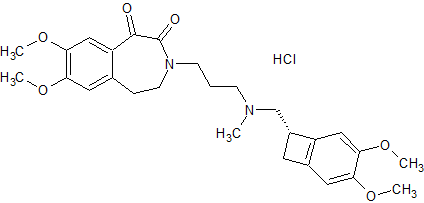
Dabigatran Impurity 21
- Product Number CT-01110-288
- Parent Drug Dabigatran
- CAS Number 211914-51-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
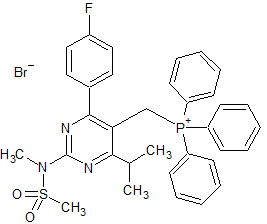
Olmesartan Impurity 29
- Product Number CT-01110-340
- Parent Drug Olmesartan
- CAS Number 76-83-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ivabradine Impurity 10
- Product Number CT-01110-397
- Parent Drug Ivabradine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
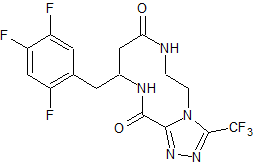
Sitagliptin impurity 29
- Product Number CT-01110-441
- Parent Drug Sitagliptin
- CAS Number 2088771-61-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Rosuvastatin Impurity 65
- Product Number CT-01110-500
- Parent Drug Rosuvastatin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tirofiban Impurity 23
- Product Number CT-01110-603
- Parent Drug Tirofiban
- CAS Number 149490-60-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options