Drug Impurities Reference Standards
Showing 1541–1550 of 1775 results
-
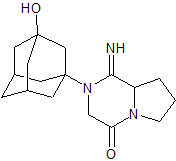
Vildagliptin Impurity 5
- Product Number CT-01110-350
- Parent Drug Vildagliptin
- CAS Number 1789703-37-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
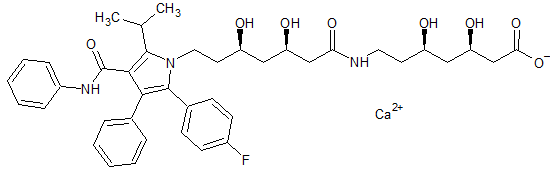
Atorvastatin EP Impurity F(Calcium Salt)
- Product Number CT-01110-420
- Parent Drug Atorvastatin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
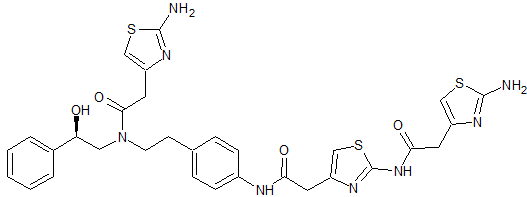
Mirabegron Impurity 5
- Product Number CT-01110-84
- Parent Drug Mirabegron
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
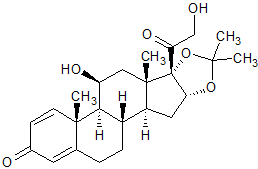
Budesonide EP Impurity F(Desonide)
- Product Number CT-01110-721
- Parent Drug Budesonide
- CAS Number 638-94-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
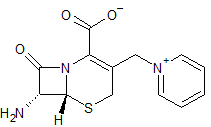
Ceftazidime EP Impurity C
- Product Number CT-01110-542
- Parent Drug Ceftazidime
- CAS Number 3432-88-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
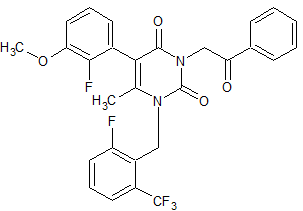
Elagolix Impurity 8
- Product Number CT-01110-1121
- Parent Drug Elagolix
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
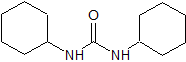
Flunarizine Impurity 8
- Product Number CT-01110-659
- Parent Drug Flunarizine
- CAS Number 365-24-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Indapamide Impurity 1
- Product Number CT-01110-740
- Parent Drug Indapamide
- CAS Number 2387-23-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
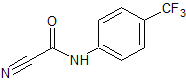
Leflunomide EP Impurity H
- Product Number CT-01110-916
- Parent Drug Leflunomide
- CAS Number 24522-30-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
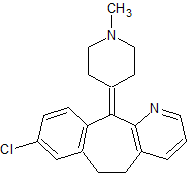
Loratadine EP Impurity G
- Product Number CT-01110-781
- Parent Drug Loratadine
- CAS Number 38092-89-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options