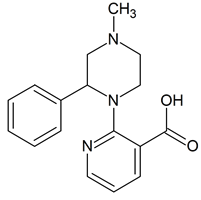Drug Impurities Reference Standards
Showing 1551–1560 of 1775 results
-
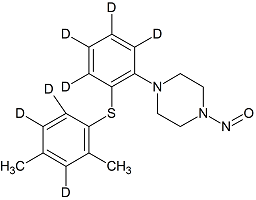
Vortioxetine-D7 N-nitroso
- Product Number V-31012-01
- Parent Drug Vortioxetine
- CAS Number N/A
- Category Drug Impurities Reference Standards
DiscontinuedSee more size options -
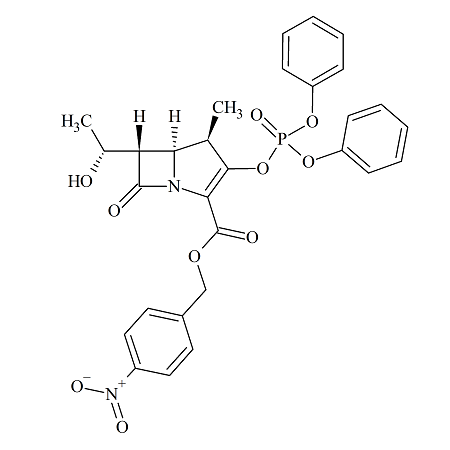
Meropenem Intermediate: 1ß-Methyl Carbapenem Derivative
- Product Number MRP-16-001
- Parent Drug Meropenem
- CAS Number 90776-59-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
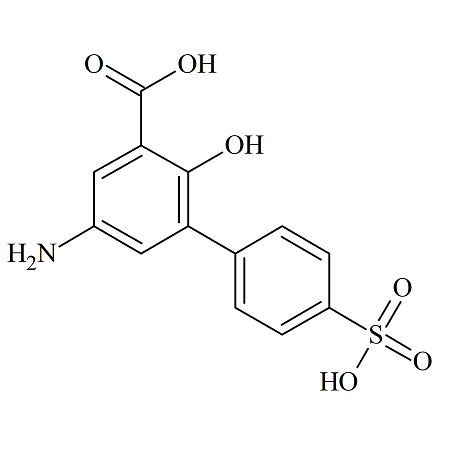
Crisaborole Impurity D
- Product Number C-01006-04
- Parent Drug Crisaborole
- CAS Number 1187188-59-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
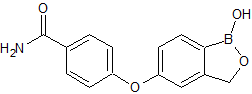
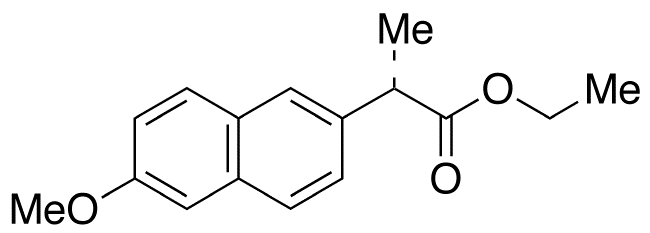
Naproxen Ethyl Ester
- Product Number NAP-16-003
- Parent Drug Naproxen
- CAS Number 31220-35-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
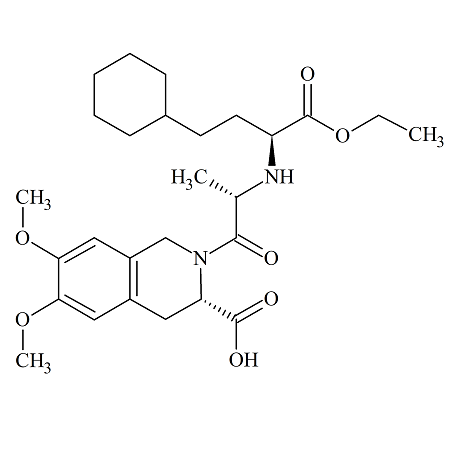
Moexipril USP RC D
- Product Number MOE-12-005
- Parent Drug Moexipril
- CAS Number 1356058-19-9
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
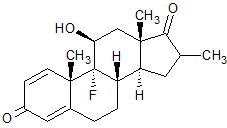
Dexamethasone 17-Ketone
- Product Number B-10125-01
- Parent Drug Dexamethasone
- CAS Number 2285-53-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
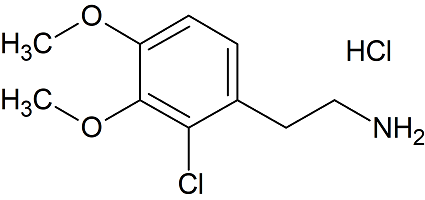
2-Chloro-3,4-dimethoxyphenethylamine hydrochloride
- Product Number D-30227-01
- Parent Drug Dopamine
- CAS Number 95413-92-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Mirtazapine Acid impurity
- Product Number M-51003-01
- Parent Drug Mirtazapine
- CAS Number 61338-13-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
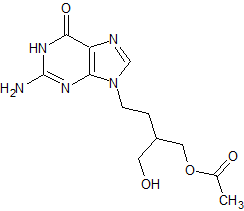
Penciclovir Monoacetate Impurity
- Product Number B-71123-0001
- Parent Drug Penciclovir
- CAS Number 97845-80-2
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options