Drug Impurities Reference Standards
Showing 1741–1750 of 1775 results
-
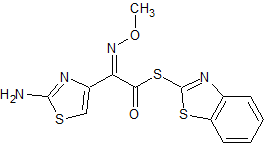
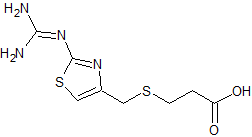
Ceftriaxone Sodium EP Impurity D
- Product Number CT-01110-469
- Parent Drug Ceftriaxone
- CAS Number 80756-85-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
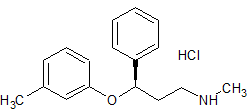
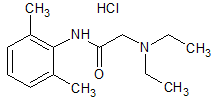
Atomoxetine EP Impurity D HCl
- Product Number CT-01110-867
- Parent Drug Atomoxetine
- CAS Number 873310-28-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
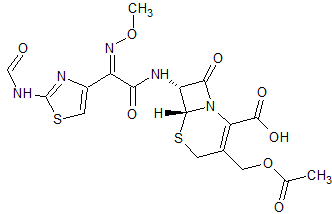
Cefotaxime EP Impurity C
- Product Number CT-01110-533
- Parent Drug Cefotaxime
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
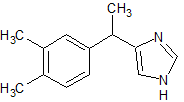
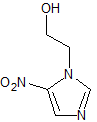
Dexmedetomidine Impurity 8
- Product Number CT-01110-554
- Parent Drug Dexmedetomidine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Famotidine EP Impurity F
- Product Number CT-01110-725
- Parent Drug Famotidine
- CAS Number 107880-74-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
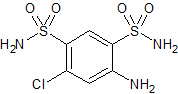
Hydrochlorothiazide EP Impurity B
- Product Number CT-01110-504
- Parent Drug Hydrochlorothiazide
- CAS Number 121-30-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
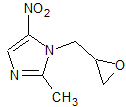
Lacosamide EP Impurity K
- Product Number CT-01110-1017
- Parent Drug Lacosamide
- CAS Number 86921-49-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Lidocaine EP Impurity F(Hydrochloride)
- Product Number CT-01110-928
- Parent Drug Lidocaine
- CAS Number 857170-72-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
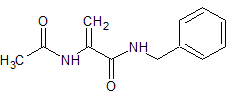
Metronidazole EP Impurity D
- Product Number CT-01110-745
- Parent Drug Metronidazole
- CAS Number 5006-68-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ornidazole Impurity 3
- Product Number CT-01110-639
- Parent Drug Ornidazole
- CAS Number 16773-52-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options