Drug Impurities Reference Standards
Showing 1731–1740 of 1775 results
-
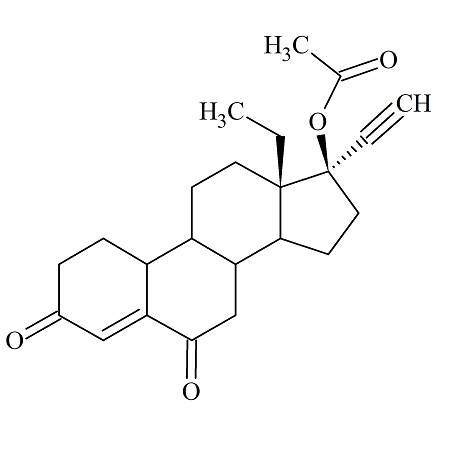
6-Keto Levonorgestrel Acetate
- Product Number ACA-160819-0010
- Parent Drug Levonorgestrel
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
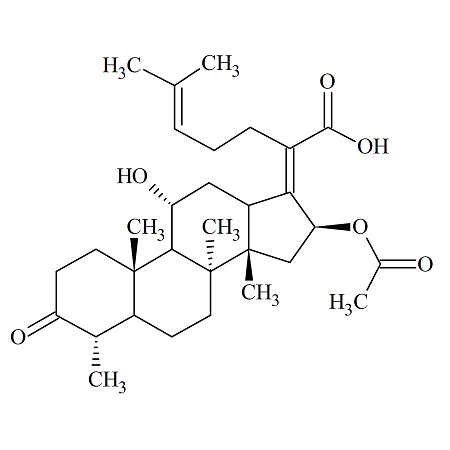
Fusidic Acid EP impurityG
- Product Number ACA-160907-0001
- Parent Drug Fusidic Acid
- CAS Number 4680-37-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
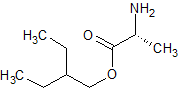
Remdesivir epi-Alanine Ester Impurity
- Product Number R-00720-09
- Parent Drug Remdesivir
- CAS Number N/A
- Category Drug Impurities Reference Standards
-
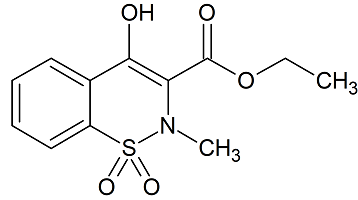
Meloxicam Related Compound A
- Product Number M-10205-01
- Parent Drug Meloxicam
- CAS Number 24683-26-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
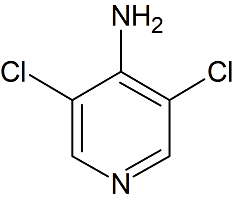
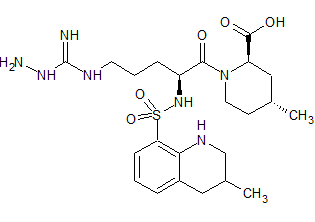
Argatroban N-Amino Impurity
- Product Number B-80309-0001
- Parent Drug Argatroban
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Linezolid Impurity 4
- Product Number CT-01110-175
- Parent Drug Linezolid
- CAS Number 496031-56-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
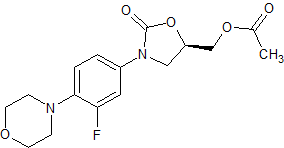
Etoricoxib Impurity 8
- Product Number CT-01110-253
- Parent Drug Etoricoxib
- CAS Number 646459-39-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
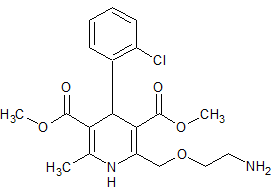
Amlodipine Besylate EP Impurity F
- Product Number CT-01110-331
- Parent Drug Amlodipine
- CAS Number 140171-66-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Acotiamide Impurity 4
- Product Number CT-01110-394
- Parent Drug Acotiamide
- CAS Number 1809272-85-2
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 3-4 week(s)See more size options