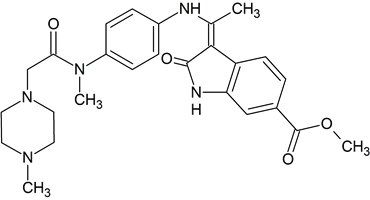Drug Impurities Reference Standards
Showing 231–240 of 1775 results
-
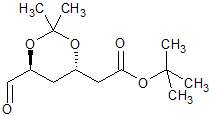
Rosuvastatin Impurity 29
- Product Number CT-01110-492
- Parent Drug Rosuvastatin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
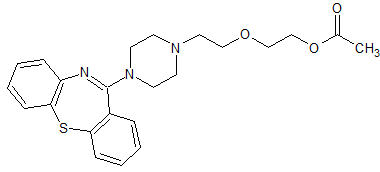
Quetiapine Impurity 22
- Product Number CT-01110-587
- Parent Drug Quetiapine
- CAS Number 844639-07-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
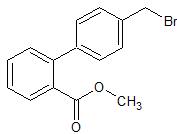
Telmisartan Impurity 12
- Product Number CT-01110-663
- Parent Drug Telmisartan
- CAS Number 114772-38-2
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
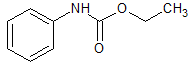
Glipizide Impurity 21
- Product Number CT-01110-751
- Parent Drug Glipizide
- CAS Number 101-99-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Indacaterol Impurity 16
- Product Number CT-01110-850
- Parent Drug Indacaterol
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
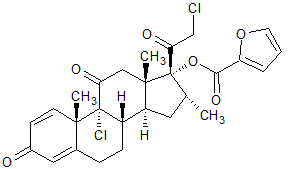
Mometasone Furoate Impurity 11
- Product Number CT-01110-953
- Parent Drug Mometasone
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Rasagiline Mesylate Impurity B
- Product Number I-30905-01
- Parent Drug Rasagiline Mesylate
- CAS Number 10277-74-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
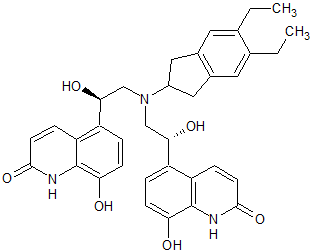
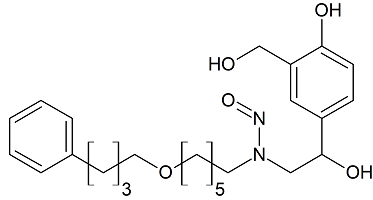
Salmeterol N-nitroso
- Product Number S-30914-01
- Parent Drug Salmeterol
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
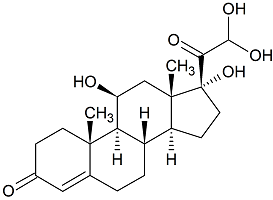
Hydrocortisone Aldehyde Hydrate
- Product Number H-20818-01
- Parent Drug Hydrocortisone
- CAS Number 906337-64-2
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Nintedanib Desphenyl
- Product Number N-40528-01
- Parent Drug Nintedanib
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options