Drug Impurities Reference Standards
Showing 201–210 of 1775 results
-
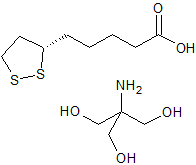
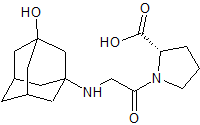
(S)-a-Lipoic Acid Tromethamine Salt
- Product Number L-91101-1
- Parent Drug Lipoic Acid
- CAS Number 248914-06-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
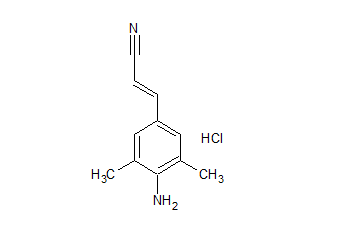
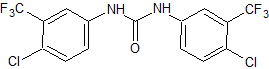
Rilpivirine Dimethylanilinoacrylonitrile Certified Impurity Reference Standard
- Product Number R-00213-05
- Parent Drug Rilpivirine
- CAS Number 661489-23-2
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
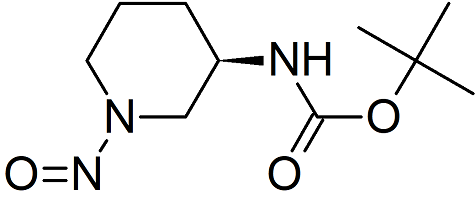
Linagliptin Impurity 4 N-Nitroso
- Product Number L-30405-01
- Parent Drug Linagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
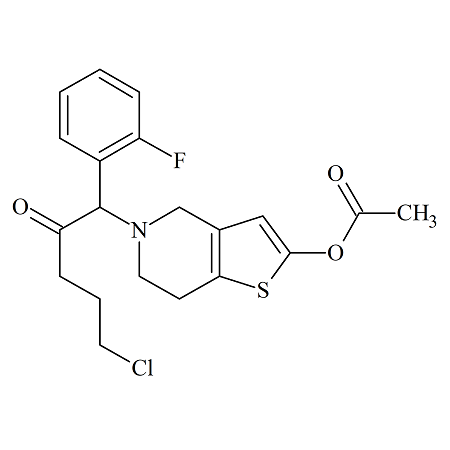
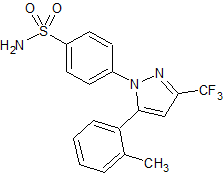
Celecoxib Impurity 8
- Product Number CT-01110-101
- Parent Drug Celecoxib
- CAS Number 170569-99-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
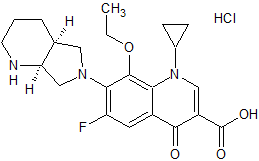
Moxifloxacin EP Impurity C
- Product Number CT-01110-192
- Parent Drug Moxifloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
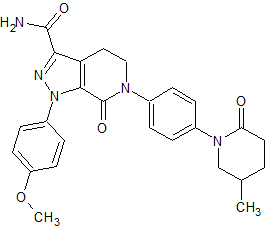
Apixaban Impurity 4 (BMS-728626-01)
- Product Number CT-01110-282
- Parent Drug Apixaban
- CAS Number 1686149-74-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vildagliptin Impurity 3
- Product Number CT-01110-348
- Parent Drug Vildagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Sorafenib Impurity 9
- Product Number CT-01110-413
- Parent Drug Sorafenib
- CAS Number 370-50-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
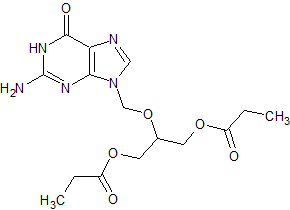
Ganciclovir EP Impurity I
- Product Number CT-01110-74
- Parent Drug Ganciclovir
- CAS Number 86357-20-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options