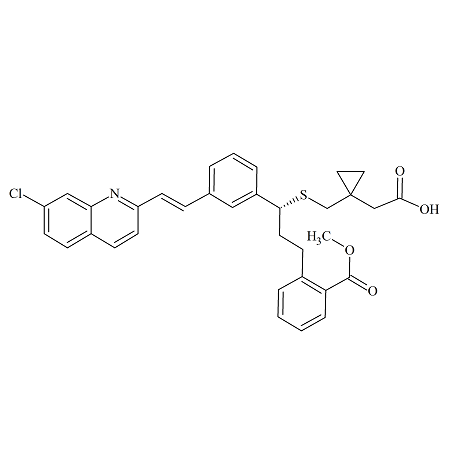
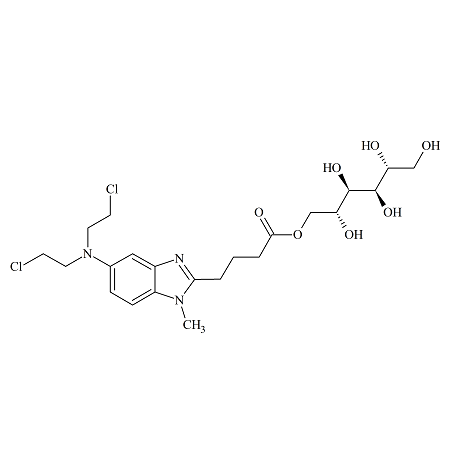
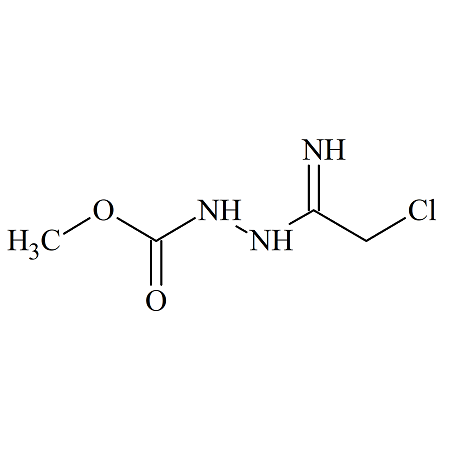
Drug Impurities Reference Standards
Showing 361–370 of 1775 results
-
Sorafenib EP Impurity C
- Product Number S-90507-03
- Parent Drug Sorafenib
- CAS Number 320-51-4
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
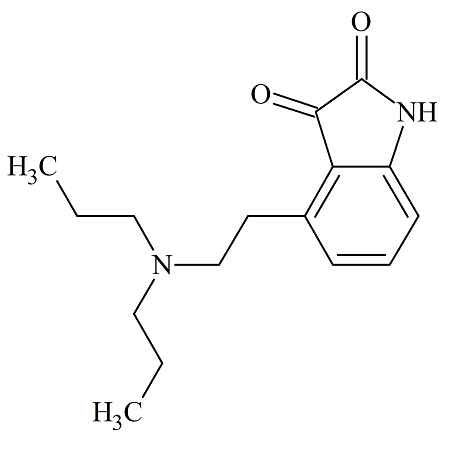
Montelukast EP Impurity H
- Product Number MON-12-002
- Parent Drug Montelukast
- CAS Number 851755-56-1
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Aprepitant Stage-III (Impurity-D)
- Product Number ACA-160919-0003
- Parent Drug Aprepitant
- CAS Number 155742-64-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
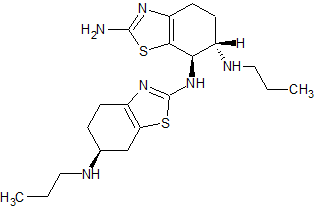
Pramipexole Dihydrochloride Impurity BI-II786BS
- Product Number P-90729-01
- Parent Drug Pramipexole
- CAS Number 1244656-98-1
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Nilotinib Related Compound A USP Impurity Certified Reference Standard
- Product Number NIL-15-002
- Parent Drug Nilotinib
- CAS Number 641571-11-1
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 week(s)See more size options -
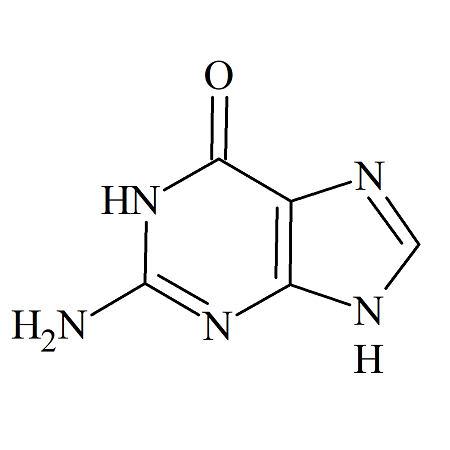
Acyclovir Impurity B
- Product Number ACA-161005-0002
- Parent Drug Acyclovir
- CAS Number 73-40-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
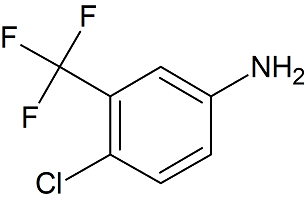
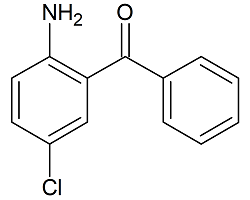
2-Amino-5-Chlorobenzophenone
- Product Number B-10208-01
- Parent Drug Benzophenone
- CAS Number 719-59-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
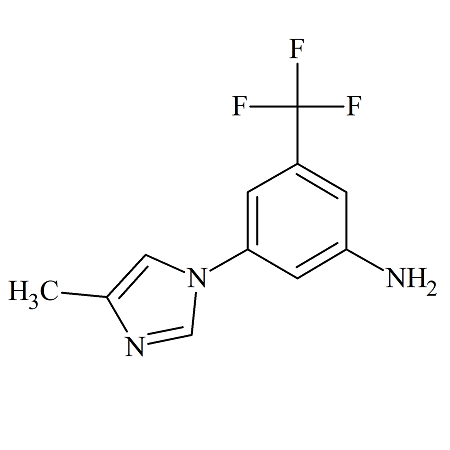
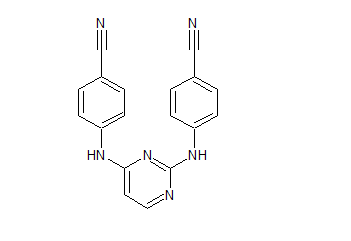
Rilpivirine Dibenzonitrile Certified Impurity Reference Standard
- Product Number R-00213-06
- Parent Drug Rilpivirine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options