Drug Impurities Reference Standards
Showing 341–350 of 1775 results
-
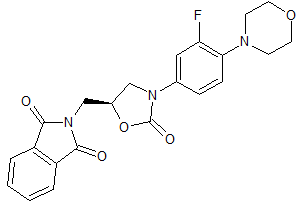
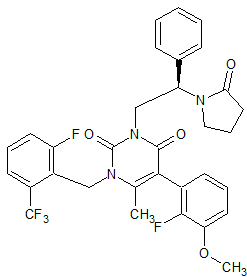
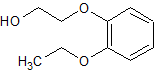
Linezolid Impurity 26
- Product Number CT-01110-171
- Parent Drug Linezolid
- CAS Number 168828-89-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
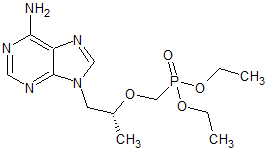
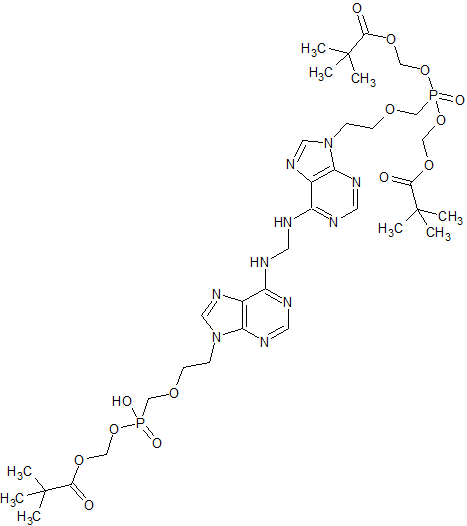
Tenofovir disoproxil Impurity 20
- Product Number CT-01110-215
- Parent Drug Tenofovir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
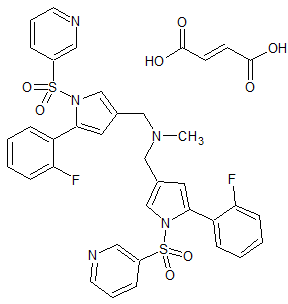
Vonoprazan Fumarate Impurity 28
- Product Number CT-01110-52
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
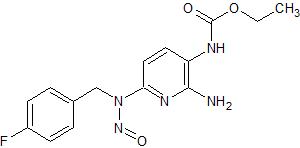
Flupirtine N-Nitroso
- Product Number F-10312-01
- Parent Drug Flupirtine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Elagolix Impurity 11
- Product Number CT-01110-1117
- Parent Drug Elagolix
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
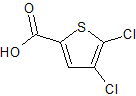
Rivaroxaban Impurity 22
- Product Number CT-01110-294
- Parent Drug Rivaroxaban
- CAS Number 31166-29-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vildagliptin Impurity 24
- Product Number CT-01110-347
- Parent Drug Vildagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Rabeprazole EP Impurity E
- Product Number CT-01110-402
- Parent Drug Rabeprazole
- CAS Number 102804-77-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
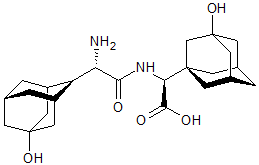
Adefovir Impurity 23
- Product Number CT-01110-455
- Parent Drug Adefovir
- CAS Number 1215101-42-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tamsulosin Impurity 11
- Product Number CT-01110-511
- Parent Drug Tamsulosin
- CAS Number 3250-73-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options