Drug Impurities Reference Standards
Showing 501–510 of 1775 results
-
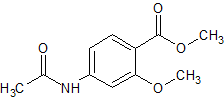
Metoclopramide EP Impurity D
- Product Number CT-01110-773
- Parent Drug Metoclopramide
- CAS Number 4093-29-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
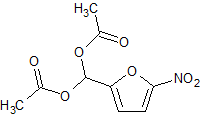
Nifuratel Impurity 1
- Product Number CT-01110-615
- Parent Drug Nifuratel
- CAS Number 92-55-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
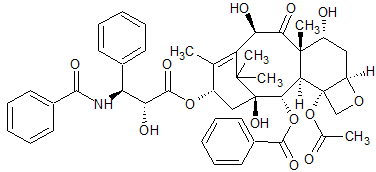
Paclitaxel EP Impurity H
- Product Number CT-01110-1035
- Parent Drug Paclitaxel
- CAS Number 78454-17-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
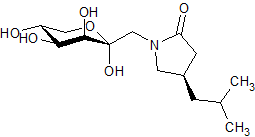
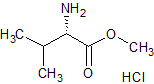
Pregabalin Impurity PD 0224377
- Product Number CT-01110-624
- Parent Drug Pregabalin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ribavirin EP Impurity C
- Product Number CT-01110-767
- Parent Drug Ribavirin
- CAS Number 4928-87-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
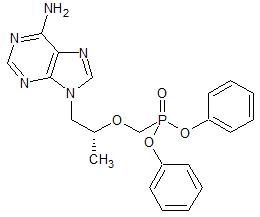
Tenofovir Alafenamide Impurity 1
- Product Number CT-01110-986
- Parent Drug Tenofovir
- CAS Number 342631-41-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Voriconazole EP Impurity C
- Product Number CT-01110-683
- Parent Drug Voriconazole
- CAS Number 137234-88-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Valsartan ImpurIty 12
- Product Number CT-01110-130
- Parent Drug Valsartan
- CAS Number 6306-52-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
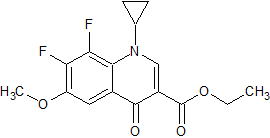
Moxifloxacin Impurity 11
- Product Number CT-01110-182
- Parent Drug Moxifloxacin
- CAS Number 1329836-33-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
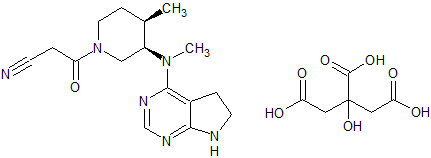
Tofacitinib Impurity 14
- Product Number CT-01110-227
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options