Drug Impurities Reference Standards
Showing 511–520 of 1775 results
-
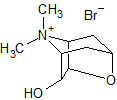
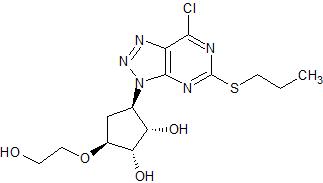
Tiotropium Bromide EP Impurity H (Mixture of Enantiomers)
- Product Number CT-01110-978
- Parent Drug Tiotropium
- CAS Number 1044148-31-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Zopiclone EP Impurity B
- Product Number CT-01110-569
- Parent Drug Zopiclone
- CAS Number 43200-81-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
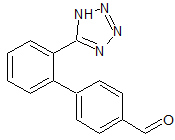
Valsartan Impurity 34
- Product Number CT-01110-136
- Parent Drug Valsartan
- CAS Number 151052-40-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
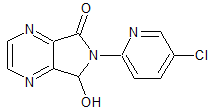
Moxifloxacin Impruty 26
- Product Number CT-01110-189
- Parent Drug Moxifloxacin
- CAS Number 452092-31-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
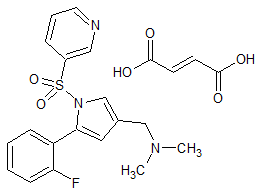
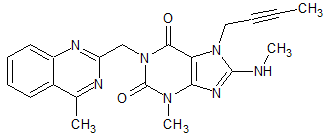
Tofacitinib Impurity 37
- Product Number CT-01110-234
- Parent Drug Tofacitinib
- CAS Number 1252883-90-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
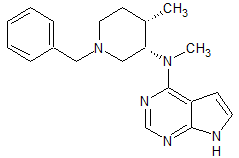
Vonoprazan Fumarate Impurity 52
- Product Number CT-01110-66
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
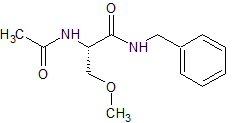
Lacosamide EP Impurity A
- Product Number CT-01110-1013
- Parent Drug Lacosamide
- CAS Number 175481-37-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
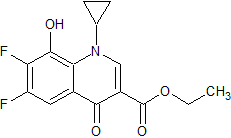
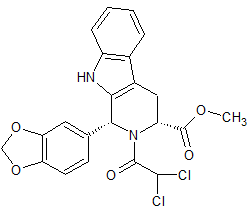
Tadalafil Impurity 26
- Product Number CT-01110-263
- Parent Drug Tadalafil
- CAS Number 1598416-08-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ticagrelor Impurity 11
- Product Number CT-01110-311
- Parent Drug Ticagrelor
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Linagliptin Impurity 47
- Product Number CT-01110-360
- Parent Drug Linagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options