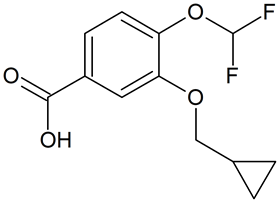Drug Impurities Reference Standards
Showing 741–750 of 1775 results
-
Gestodene Impurity E
- Product Number ACB-161030-0031
- Parent Drug Gestodene
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
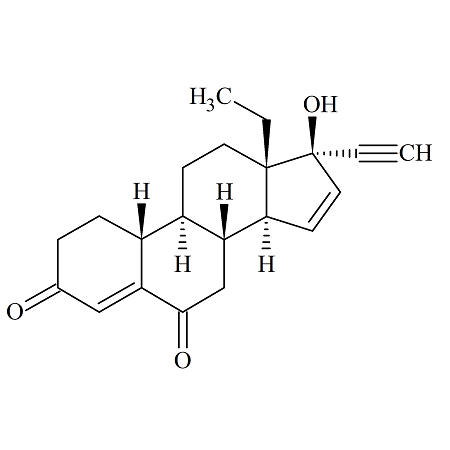
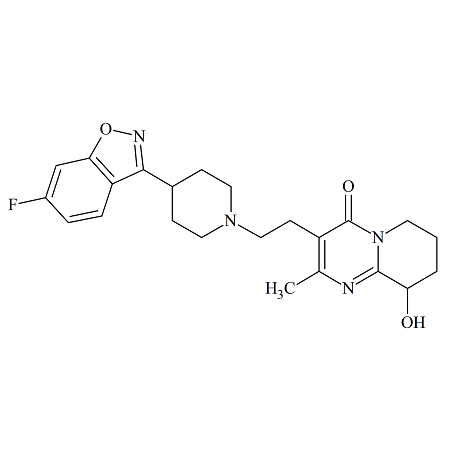
4’-Hydroxy Dutasteride
- Product Number ACA-160819-0022
- Parent Drug Dutasteride
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
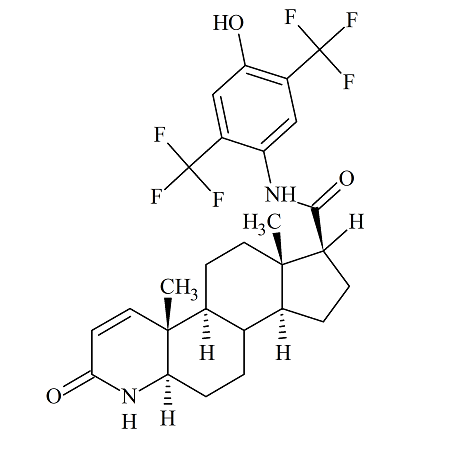
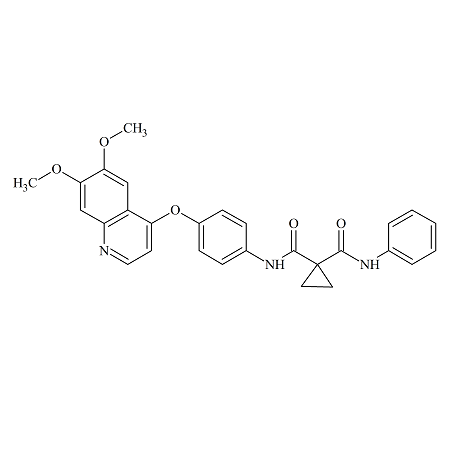
Cabozantinib Desfluoro
- Product Number B-70831-3
- Parent Drug Cabozantinib
- CAS Number 849221-94-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
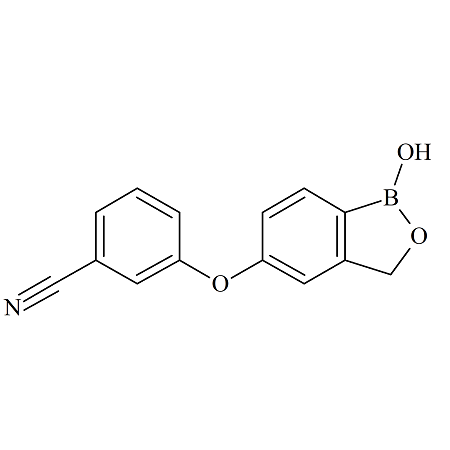
Crisaborole m-Isomer
- Product Number ACB-170828-0003
- Parent Drug Crisaborole
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
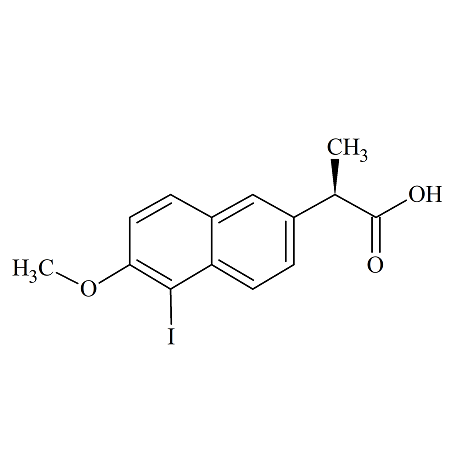
O-Iodo Naproxen
- Product Number NAP-16-005
- Parent Drug Naproxen
- CAS Number 116883-61-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
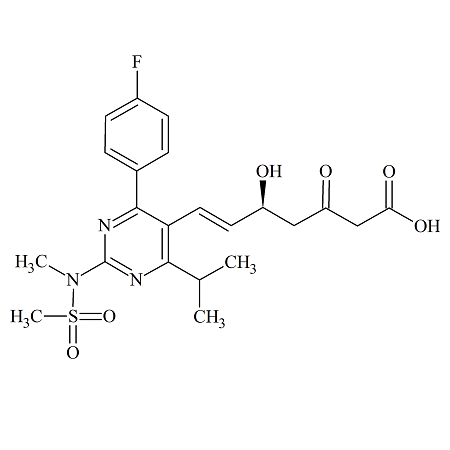
Rosuvastatin 3-Keto Acid
- Product Number ROS-12-004
- Parent Drug Rosuvastatin
- CAS Number 1346747-49-6
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
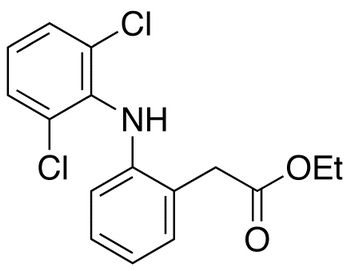
Diclofenac Ethyl Ester
- Product Number DIC-16-003
- Parent Drug Diclofenac
- CAS Number 15307-77-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
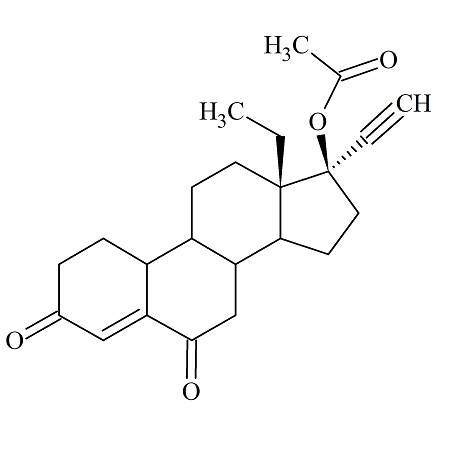
6-Keto Levonorgestrel Acetate
- Product Number ACA-160819-0010
- Parent Drug Levonorgestrel
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options