Drug Impurities Reference Standards
Showing 731–740 of 1775 results
-
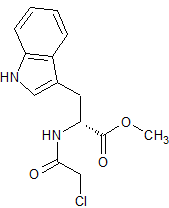
Tadalafil Impurity 41
- Product Number CT-01110-270
- Parent Drug Tadalafil
- CAS Number 185750-07-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
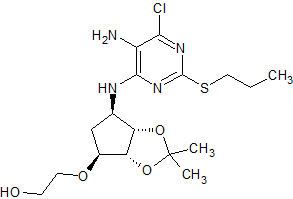
Ticagrelor Impurity 47
- Product Number CT-01110-318
- Parent Drug Ticagrelor
- CAS Number 376608-74-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
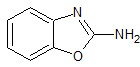
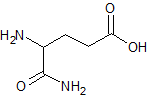
Pramipexole Impurity 14
- Product Number CT-01110-373
- Parent Drug Pramipexole
- CAS Number 4570-41-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
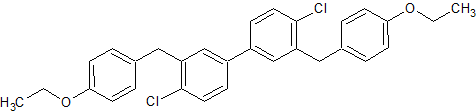
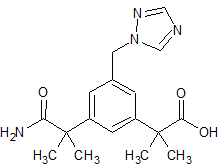
Dapagliflozin Impurity 24
- Product Number CT-01110-429
- Parent Drug Dapagliflozin
- CAS Number 2176485-21-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
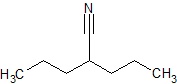
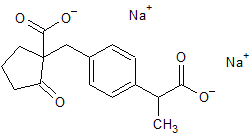
Sodium Valproate Impurity 20
- Product Number CT-01110-481
- Parent Drug Valproic Acid
- CAS Number 13310-75-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
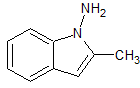
Anastrozole Impurity 15
- Product Number CT-01110-572
- Parent Drug Anastrozole
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pomalidomide Impurity 13
- Product Number CT-01110-652
- Parent Drug Pomalidomide
- CAS Number 636-65-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Indapamide Impurity 20
- Product Number CT-01110-741
- Parent Drug Indapamide
- CAS Number 53406-41-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Loxoprofen Impurity 37
- Product Number CT-01110-841
- Parent Drug Loxoprofen
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
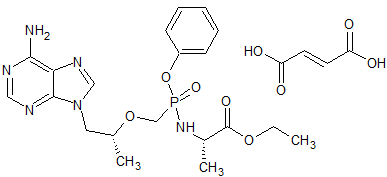
Tenofovir alafenamide Impurity 17
- Product Number CT-01110-990
- Parent Drug Tenofovir
- CAS Number 390409-48-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options