Drug Impurities Reference Standards
Showing 791–800 of 1775 results
-
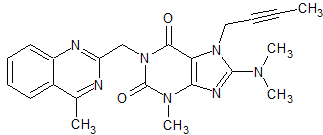
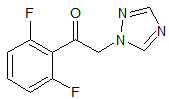
Linagliptin Impurity 10
- Product Number CT-01110-351
- Parent Drug Linagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
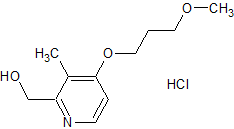
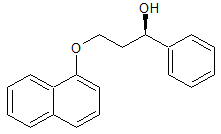
Rabeprazole Impurity 22
- Product Number CT-01110-404
- Parent Drug Rabeprazole
- CAS Number 675198-19-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
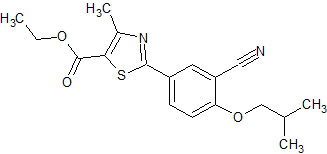
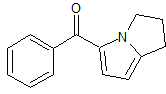
Febuxostat Impurity 13
- Product Number CT-01110-461
- Parent Drug Febuxostat
- CAS Number 160844-75-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
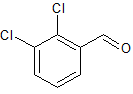
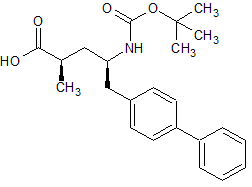
Captopril EP Impurity C
- Product Number CT-01110-515
- Parent Drug Captopril
- CAS Number 26473-47-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ixazomib Impurity 13
- Product Number CT-01110-609
- Parent Drug Ixazomib
- CAS Number 6334-18-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Voriconazole Impurity 31
- Product Number CT-01110-684
- Parent Drug Voriconazole
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Dapoxetine Impurity 19
- Product Number CT-01110-795
- Parent Drug Dapoxetine
- CAS Number 156453-53-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ketorolac EP Impurity I
- Product Number CT-01110-909
- Parent Drug Ketorolac
- CAS Number 113502-55-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
LCZ-696 Impurity 18(S,R)
- Product Number CT-01110-942
- Parent Drug LCZ-696
- CAS Number 1012341-54-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
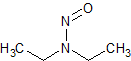
N-nitrosodiethylamine (NDEA)
- Product Number N-10521-03
- Parent Drug Nitroso Compounds
- CAS Number 55-18-5
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options