Drug Impurities Reference Standards
Showing 821–830 of 1775 results
-
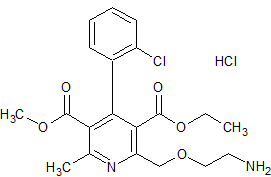
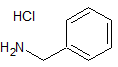
Amlodipine Besylate EP Impurity D Hydrochloride
- Product Number CT-01110-330
- Parent Drug Amlodipine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Lansoprazole Impurity 8
- Product Number CT-01110-392
- Parent Drug Lansoprazole
- CAS Number 127337-60-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
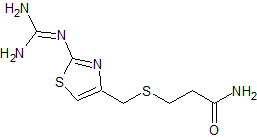
Ceftriaxone Sodium EP Impurity B(Cefotaxime EP Impurity E)(Cefodizime Impurity D)
- Product Number CT-01110-468
- Parent Drug Ceftriaxone
- CAS Number 66340-33-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
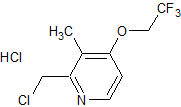
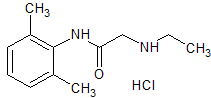
Atomoxetine EP Impurity C HCl
- Product Number CT-01110-866
- Parent Drug Atomoxetine
- CAS Number 1643684-06-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Cefminox Impurity 1
- Product Number CT-01110-1082
- Parent Drug Cefminox
- CAS Number 13183-79-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Dexmedetomidine Impurity 2
- Product Number CT-01110-552
- Parent Drug Dexmedetomidine
- CAS Number 5779-93-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Famotidine EP Impurity D
- Product Number CT-01110-724
- Parent Drug Famotidine
- CAS Number 76824-16-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
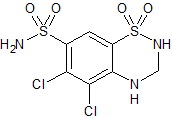
Hydrochlorothiazide 5-Chloro Impurity
- Product Number CT-01110-505
- Parent Drug Hydrochlorothiazide
- CAS Number 5233-42-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Lacosamide EP Impurity J HCl
- Product Number CT-01110-1016
- Parent Drug Lacosamide
- CAS Number 3287-99-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Lidocaine EP Impurity D(HCl)
- Product Number CT-01110-927
- Parent Drug Lidocaine
- CAS Number 7729-94-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options