Drug Impurities Reference Standards
Showing 1181–1190 of 1775 results
-
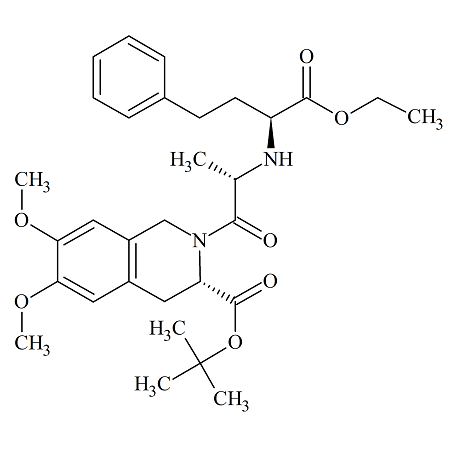
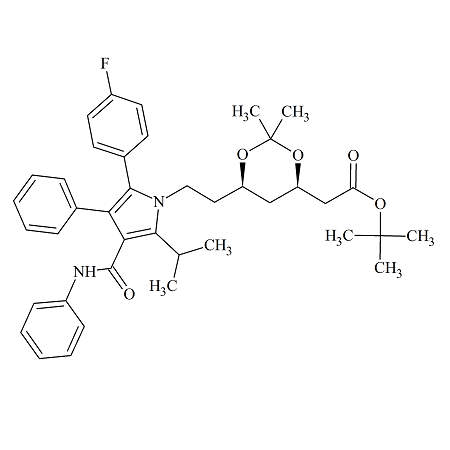
Moexipril USP RC C
- Product Number MOE-12-004
- Parent Drug Moexipril
- CAS Number 103733-39-7
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
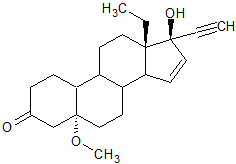
Gestodene Impurity I
- Product Number G-90916-02
- Parent Drug Gestodene
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
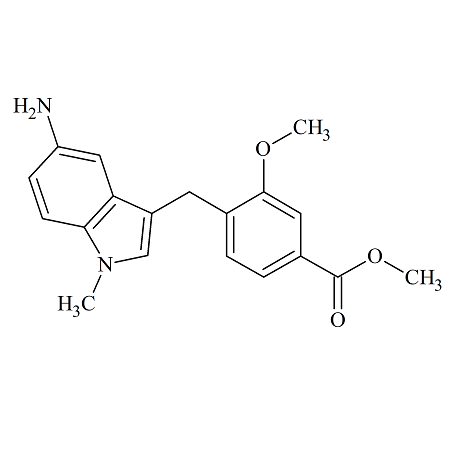
Zafirlukast 5-Aminoindole Impurity
- Product Number ACB-170824-0004
- Parent Drug Zafirlukast
- CAS Number 107754-14-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Olanzapine Related Compound A
- Product Number O-10208-01
- Parent Drug Olanzapine
- CAS Number 138564-59-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
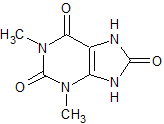
Aminophylline EP ImpurityE
- Product Number CT-01110-1044
- Parent Drug Aminophylline
- CAS Number 944-73-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
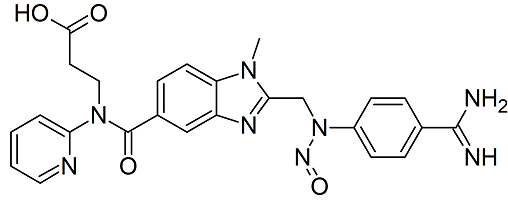
Dabigatran N-nitroso
- Product Number D-30823-01
- Parent Drug Dabigatran
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Remdesivir Phenyl Phosphate Impurity
- Product Number R-00720-03
- Parent Drug Remdesivir
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
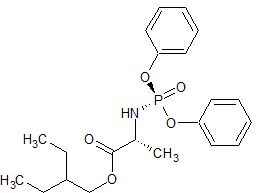
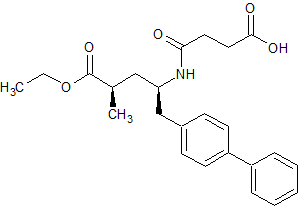
Sacubitril-(2R,4R)-Isomer
- Product Number S-90710-01
- Parent Drug Sacubitril
- CAS Number 766480-48-2
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
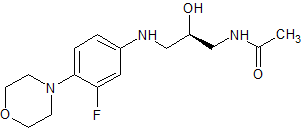
Linezolid Impurity 6
- Product Number CT-01110-179
- Parent Drug Linezolid
- CAS Number 333753-67-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options