Drug Impurities Reference Standards
Showing 1211–1220 of 1775 results
-
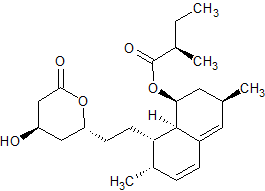
Simvastatin EP Impurity F
- Product Number CT-01110-531
- Parent Drug Simvastatin
- CAS Number 79952-44-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
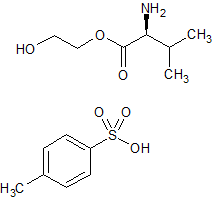
Valaciclovir EP Impurity F(p-toluenesulfonic acid)
- Product Number CT-01110-597
- Parent Drug Valaciclovir
- CAS Number 86150-60-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tedizolid Impurity 31
- Product Number CT-01110-117
- Parent Drug Tedizolid
- CAS Number 1239662-46-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Levetiracetam Impurity 18
- Product Number CT-01110-159
- Parent Drug Levetiracetam
- CAS Number 627-00-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Moxifloxacin Impurity 60
- Product Number CT-01110-206
- Parent Drug Moxifloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
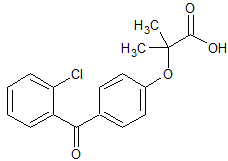
2-Chloro Fenofibric Acid
- Product Number CT-01110-41
- Parent Drug Fenofibrate
- CAS Number 61024-31-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Celecoxib Impurity 11
- Product Number CT-01110-94
- Parent Drug Celecoxib
- CAS Number 219986-64-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
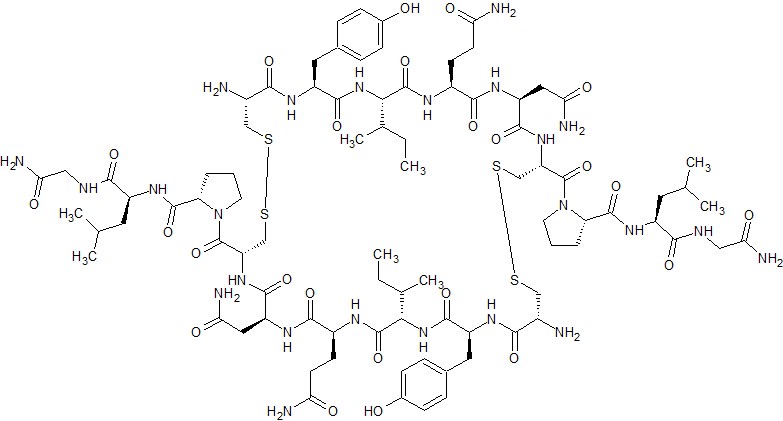
Beta-Oxytocin Dimer
- Product Number CT-01110-1104
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Dabigatran Impurity 15
- Product Number CT-01110-286
- Parent Drug Dabigatran
- CAS Number 1702936-92-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
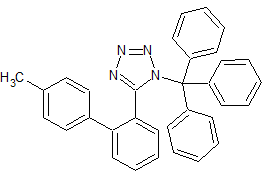
Olmesartan Impurity 16
- Product Number CT-01110-338
- Parent Drug Olmesartan
- CAS Number 124750-53-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options