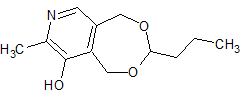
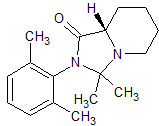
Drug Impurities Reference Standards
Showing 141–150 of 1775 results
-
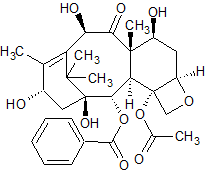
Docetaxel EP Impurity E
- Product Number CT-01110-897
- Parent Drug Docetaxel
- CAS Number 32981-86-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
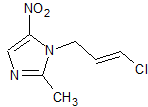
Fasudil Impurity 3
- Product Number CT-01110-677
- Parent Drug Fasudil
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
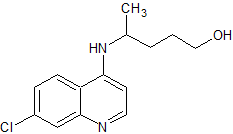
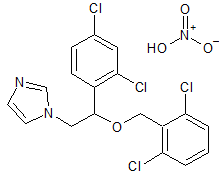
Hydroxychloroquine EP Impurity E
- Product Number CT-01110-1022
- Parent Drug Hydroxychloroquine
- CAS Number 10500-64-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
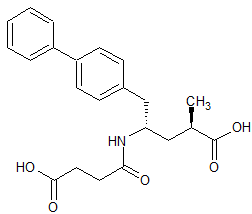
LCZ-696 Impurity 1
- Product Number CT-01110-939
- Parent Drug LCZ-696
- CAS Number 149709-44-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
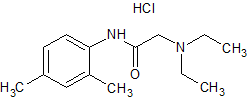
Lidocaine EP Impurity I
- Product Number CT-01110-931
- Parent Drug Lidocaine
- CAS Number 17289-54-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Miconazole EP Impurity D
- Product Number CT-01110-995
- Parent Drug Miconazole
- CAS Number 24168-96-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ornidazole Impurity 8
- Product Number CT-01110-642
- Parent Drug Ornidazole
- CAS Number 1384752-15-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pimavanserin Impurity 7
- Product Number CT-01110-1090
- Parent Drug Pimavanserin
- CAS Number 1035343
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pyridoxine Impurity 7
- Product Number CT-01110-1049
- Parent Drug Pyridoxine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ropivacaine EP Impurity F
- Product Number CT-01110-973
- Parent Drug Ropivacaine
- CAS Number 1945965-95-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options