Drug Impurities Reference Standards
Showing 1271–1280 of 1775 results
-
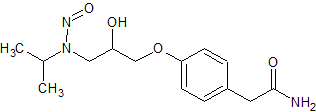
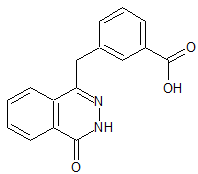
Atenolol N-Nitroso
- Product Number A-10521-03
- Parent Drug Atenolol
- CAS Number 134720-04-0
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
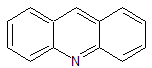
Tadalafil Impurity 31
- Product Number CT-01110-266
- Parent Drug Tadalafil
- CAS Number 2058231-08-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
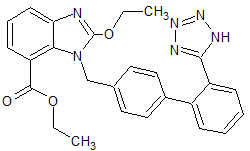
Ticagrelor impurity 21
- Product Number CT-01110-313
- Parent Drug Ticagrelor
- CAS Number 1402222-66-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
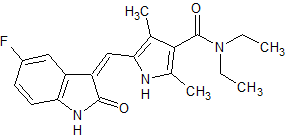
Linagliptin Impurity 49
- Product Number CT-01110-362
- Parent Drug Linagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
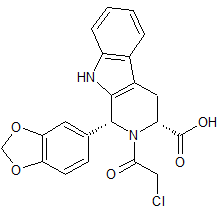
Olaparib Impurity 14
- Product Number CT-01110-422
- Parent Drug Olaparib
- CAS Number 420846-72-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Oxcarbazepine Impurity 20
- Product Number CT-01110-476
- Parent Drug Oxcarbazepine
- CAS Number 260-94-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
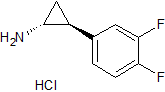
Candesartan Cilexetil EP Impurity A
- Product Number CT-01110-557
- Parent Drug Candesartan
- CAS Number 139481-58-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
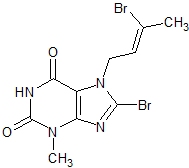
Sunitinib Impurity 25
- Product Number CT-01110-646
- Parent Drug Sunitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
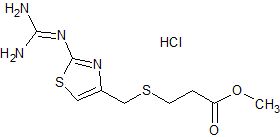
Famotidine EP Impurity J HCl
- Product Number CT-01110-722
- Parent Drug Famotidine
- CAS Number 1798006-30-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
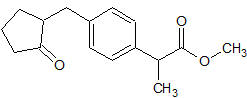
Loxoprofen Impurity 11
- Product Number CT-01110-836
- Parent Drug Loxoprofen
- CAS Number 81762-92-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options