Drug Impurities Reference Standards
Showing 1251–1260 of 1775 results
-
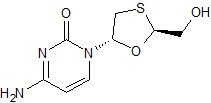
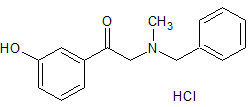
Lamivudine EP Impurity B(Enantiomer Mixture)
- Product Number CT-01110-517
- Parent Drug Lamivudine
- CAS Number 136846-20-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
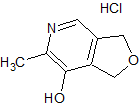
Lidocaine EP Impurity G
- Product Number CT-01110-929
- Parent Drug Lidocaine
- CAS Number 35891-87-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Isoconazole EP Impurity A ( Miconazole EP Impurity A )
- Product Number CT-01110-821
- Parent Drug Miconazole
- CAS Number 24155-42-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ornidazole Impurity 4
- Product Number CT-01110-640
- Parent Drug Ornidazole
- CAS Number 62580-80-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Phenylephrine EP Impurity E
- Product Number CT-01110-819
- Parent Drug Phenylephrine
- CAS Number 71786-67-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Pyridoxine EP Impurity A
- Product Number CT-01110-1050
- Parent Drug Pyridoxine
- CAS Number 1006-21-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
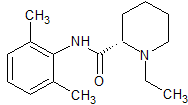
Ropivacaine EP Impurity D
- Product Number CT-01110-971
- Parent Drug Ropivacaine
- CAS Number 98626-59-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
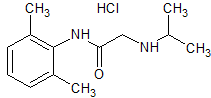
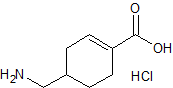
Tranexamic Acid EP Impurity C HCl
- Product Number CT-01110-980
- Parent Drug Tranexamic Acid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
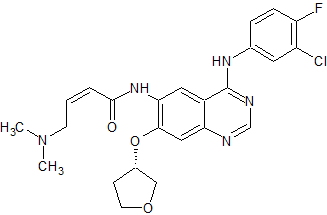
Afatinib Impurity 12
- Product Number CT-01110-04
- Parent Drug Afatinib
- CAS Number 1680184-59-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
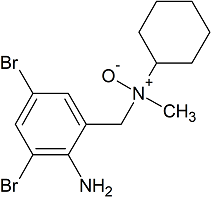
Bromhexine Impurity 15
- Product Number CT-01110-142
- Parent Drug Bromhexine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 3-4 week(s)See more size options