Drug Impurities Reference Standards
Showing 1241–1250 of 1775 results
-
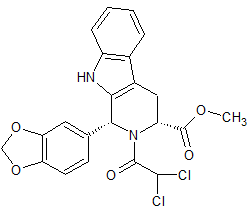
Tadalafil Impurity 26
- Product Number CT-01110-263
- Parent Drug Tadalafil
- CAS Number 1598416-08-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
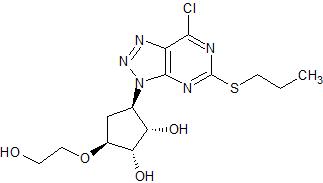
Ticagrelor Impurity 11
- Product Number CT-01110-311
- Parent Drug Ticagrelor
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
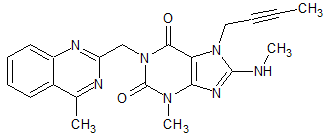
Linagliptin Impurity 47
- Product Number CT-01110-360
- Parent Drug Linagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
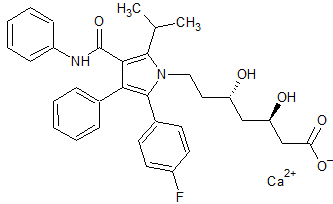
Atorvastatin Impurity 51
- Product Number CT-01110-419
- Parent Drug Atorvastatin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
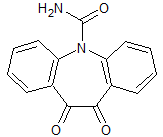
Oxcarbazepine EP Impurity I
- Product Number CT-01110-474
- Parent Drug Oxcarbazepine
- CAS Number 537693-29-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
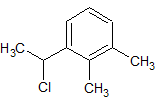
Dexmedetomidine Impurity 16
- Product Number CT-01110-551
- Parent Drug Dexmedetomidine
- CAS Number 60907-88-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
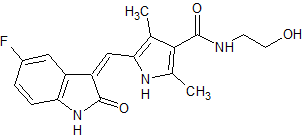
Sunitinib Impurity 21
- Product Number CT-01110-644
- Parent Drug Sunitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Levofloxacin Impurity 32
- Product Number CT-01110-707
- Parent Drug Levofloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
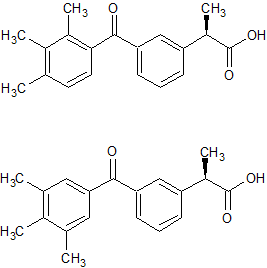
Ketoprofen EP Impurity K(And Their Enantiomers)
- Product Number CT-01110-823
- Parent Drug Ketoprofen
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
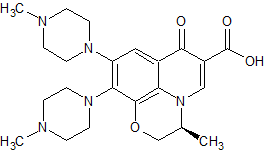
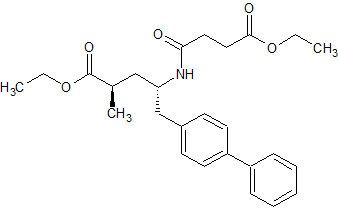
LCZ-696 Impurity 13
- Product Number CT-01110-940
- Parent Drug LCZ-696
- CAS Number 2376611-98-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options