Drug Impurities Reference Standards
Showing 1701–1710 of 1775 results
-
Nicorandil EP Impurity A
- Product Number CT-01110-1063
- Parent Drug Nicorandil
- CAS Number 65141-47-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
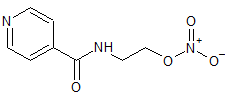
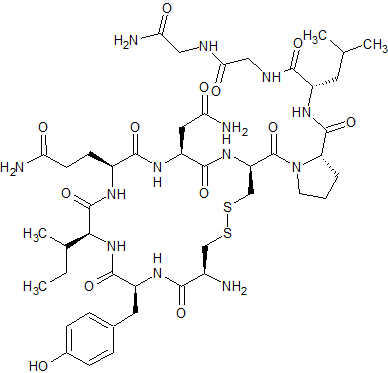
Di-Gly9-Oxytocin
- Product Number CT-01110-1110
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pomalidomide Impurity 5
- Product Number CT-01110-654
- Parent Drug Pomalidomide
- CAS Number 5434-20-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
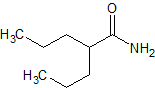
Rasagiline Impurity 9
- Product Number CT-01110-1073
- Parent Drug Rasagiline
- CAS Number 1312077-04-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tamsulosin EP Impurity I
- Product Number CT-01110-513
- Parent Drug Tamsulosin
- CAS Number 496433
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
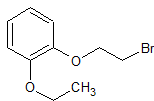
Sodium Valproate EP Impurity F
- Product Number CT-01110-479
- Parent Drug Valproic Acid
- CAS Number 2430-27-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
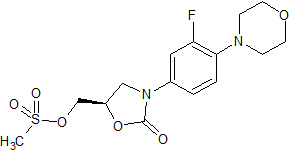
Tedizolid Impurity 40
- Product Number CT-01110-122
- Parent Drug Tedizolid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Linezolid Impurity 22
- Product Number CT-01110-170
- Parent Drug Linezolid
- CAS Number 174649-09-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
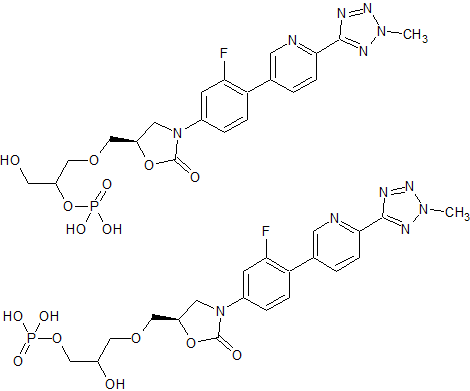
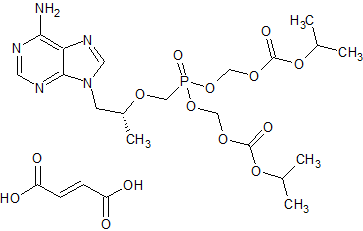
Tenofovir disoproxil Impurity 17
- Product Number CT-01110-213
- Parent Drug Tenofovir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
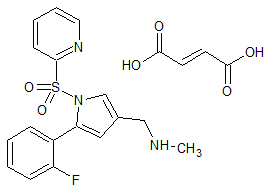
Vonoprazan Fumarate Impurity 23
- Product Number CT-01110-51
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options