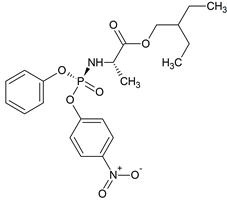Drug Impurities Reference Standards
Showing 311–320 of 1775 results
-
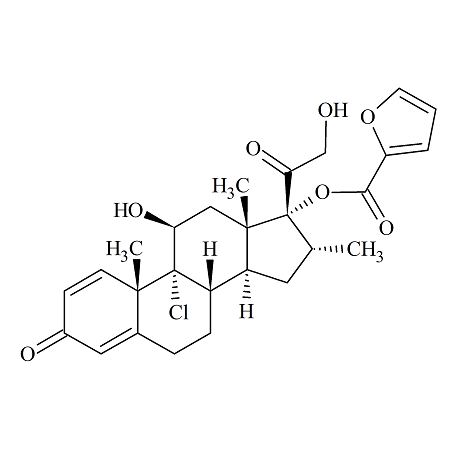
Mometasone furoate impurity; Mometasone Deschloro-Hydroxy Impurity
- Product Number MOM-14-002
- Parent Drug Mometasone
- CAS Number 148596-90-1
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 3 week(s)See more size options -
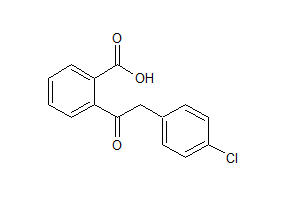
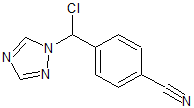
Crisaborole Process Impurity 2
- Product Number C-01120-02
- Parent Drug Crisaborole
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Paricalcitol 22-Z
- Product Number PAR-16-001
- Parent Drug Paricalcitol
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Letrozole Impurity 14
- Product Number CT-01110-1007
- Parent Drug Letrozole
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
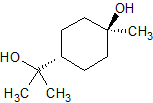
Trans-Terpin
- Product Number T-10212-01
- Parent Drug Terpin
- CAS Number 565-50-4
- Category Drug Impurities Reference Standards
DiscontinuedSee more size options -
Remdesivir Nitrophenyl Phosphate Impurity
- Product Number R-00720-04
- Parent Drug Remdesivir
- CAS Number 1354823-36-1
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
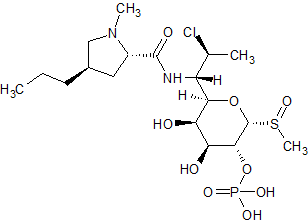
Clindamycin 2-Phosphate Sulfoxide Isomer A
- Product Number CLI-15-005-A
- Parent Drug Clindamycin
- CAS Number 1228573-90-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
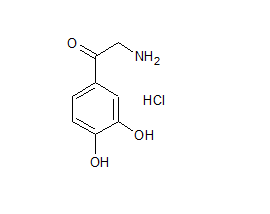
Noradrenalone HCl
- Product Number N-90111-01
- Parent Drug Norepinephrine
- CAS Number 5090-29-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Linezolid Impurity 8
- Product Number CT-01110-180
- Parent Drug Linezolid
- CAS Number 556801-15-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options