Drug Impurities Reference Standards
Showing 321–330 of 1775 results
-
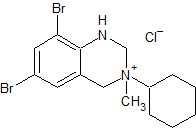
Bromhexine EP Impurity E
- Product Number CT-01110-144
- Parent Drug Bromhexine
- CAS Number 1660957-93-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
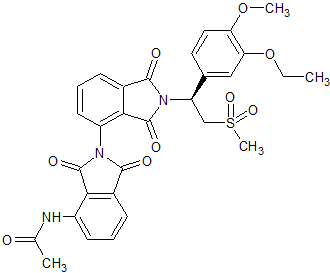
Apremilast Impurity 3
- Product Number CT-01110-22
- Parent Drug Apremilast
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
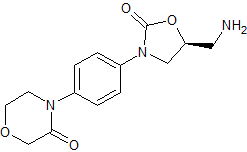
Rivaroxaban Impurity 4
- Product Number CT-01110-300
- Parent Drug Rivaroxaban
- CAS Number 446292-10-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
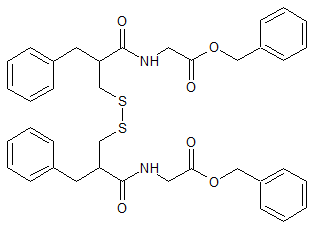
Racecadotril EP Impurity H
- Product Number CT-01110-370
- Parent Drug Racecadotril
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
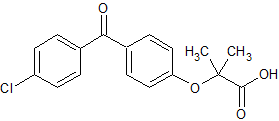
Choline Fenofibrate EP Impurity B
- Product Number CT-01110-44
- Parent Drug Fenofibrate
- CAS Number 42017-89-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
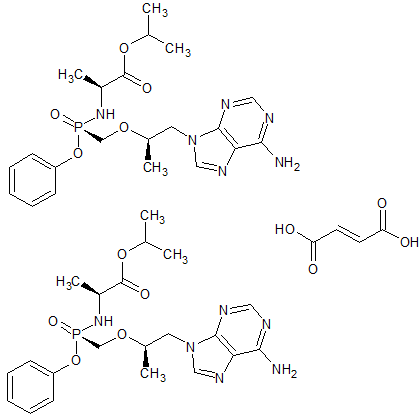
Tenofovir Alafenamide Hemifumarate
- Product Number CT-01110-985
- Parent Drug Tenofovir
- CAS Number 1392275-56-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
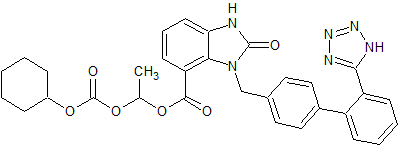
Candesartan Cilexetil EP Impurity B
- Product Number CT-01110-560
- Parent Drug Candesartan
- CAS Number 869631-11-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
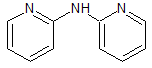
Chlorphenamine EP Impurity B
- Product Number CT-01110-514
- Parent Drug Chlorphenamine
- CAS Number 1202-34-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Eltrombopag Impurity 9
- Product Number CT-01110-527
- Parent Drug Eltrombopag
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Fluticasone Propionate EP Impurity G
- Product Number CT-01110-901
- Parent Drug Fluticasone
- CAS Number 220589-37-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options