Drug Impurities Reference Standards
Showing 571–580 of 1775 results
-
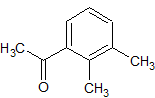
Dexmedetomidine Impurity 31
- Product Number CT-01110-553
- Parent Drug Dexmedetomidine
- CAS Number 2142-71-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
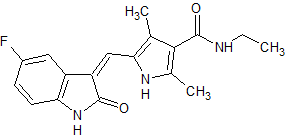
Sunitinib Impurity 24
- Product Number CT-01110-645
- Parent Drug Sunitinib
- CAS Number 1467015-10-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
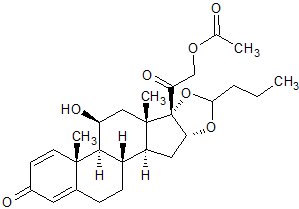
Budesonide EP Impurity K
- Product Number CT-01110-719
- Parent Drug Budesonide
- CAS Number 51333-05-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
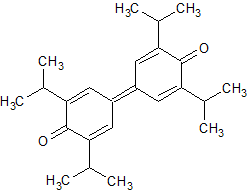
Propofol Impurity 20
- Product Number CT-01110-827
- Parent Drug Propofol
- CAS Number 2178-51-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
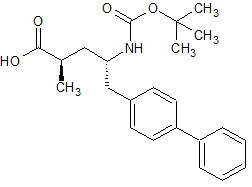
LCZ-696 Impurity 17(S,S)
- Product Number CT-01110-941
- Parent Drug LCZ-696
- CAS Number 1012341-52-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
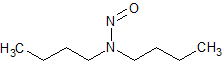
N-nitrosodibutylamine (NDBA)
- Product Number N-10521-02
- Parent Drug Nitroso Compounds
- CAS Number 924-16-3
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
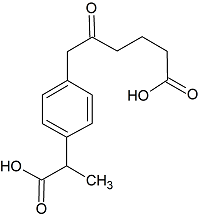
Loxoprofen Open-Ring Impurity
- Product Number L-20301-01
- Parent Drug Loxoprofen
- CAS Number 1091621-61-2
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: week(s)See more size options -
Clindamycin N-oxide
- Product Number C-401301-03
- Parent Drug Clindamycin Phosphate
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Amiodarone Hydrochloride
- Product Number A-41127-01
- Parent Drug Amiodarone
- CAS Number 19774-82-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options