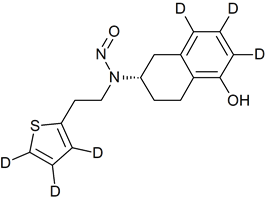Drug Impurities Reference Standards
Showing 581–590 of 1775 results
-
Ketorolac EP Impurity A
- Product Number CT-01110-908
- Parent Drug Ketorolac
- CAS Number 154476-25-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
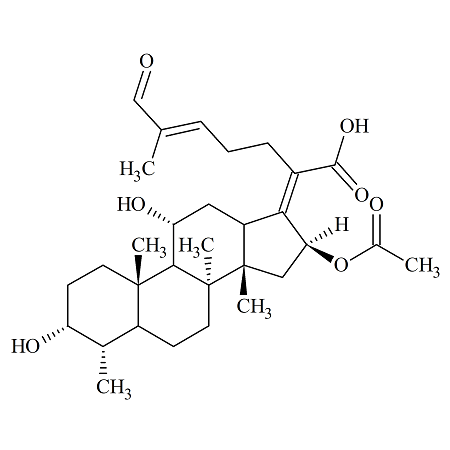
Fusidic Acid EP Impurity F
- Product Number FUS-14-002
- Parent Drug Fusidic Acid
- CAS Number 1415035-94-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
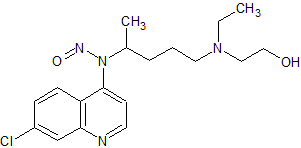
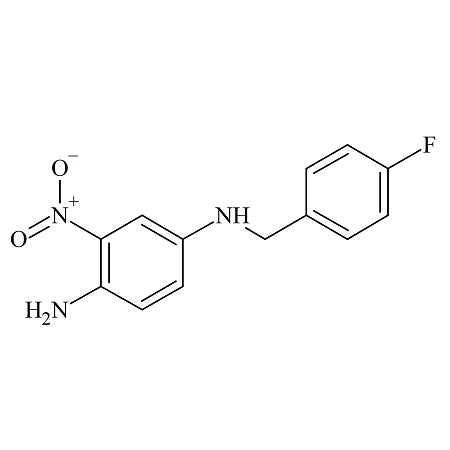
Hydroxychloroquine N-Nitroso
- Product Number H-10521-01
- Parent Drug Hydroxychloroquine
- CAS Number N/A
- Category Drug Impurities Reference Standards
DiscontinuedSee more size options -
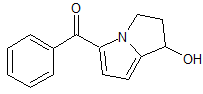
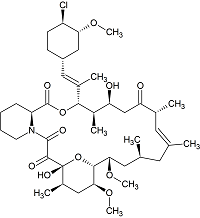
Desmethyl Pimecrolimus
- Product Number P-20126-01
- Parent Drug Pimecrolimus
- CAS Number 1025113-89-6
- Category Drug Impurities Reference Standards
DiscontinuedSee more size options -
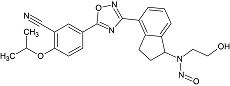
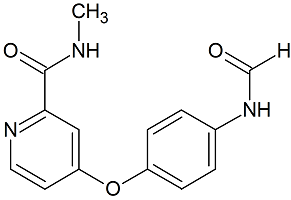
Ozanimod N-Nitroso
- Product Number O-21017-01
- Parent Drug Ozanimod
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Rotigotine USP RC C-D6 N-nitroso
- Product Number R-40821-01
- Parent Drug Rotigotine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
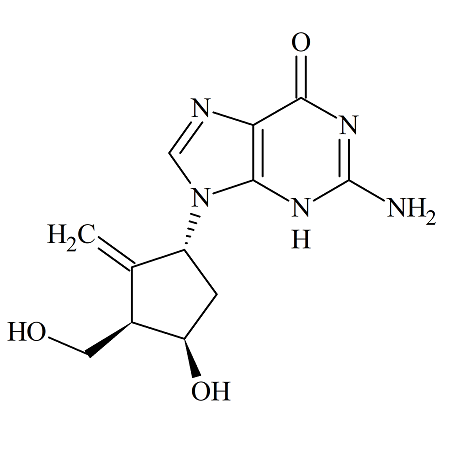
Entecavir (1R, 3R, 4R) Diastereomer
- Product Number ACA-161006-0011
- Parent Drug Entecavir
- CAS Number 1367369-76-3
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
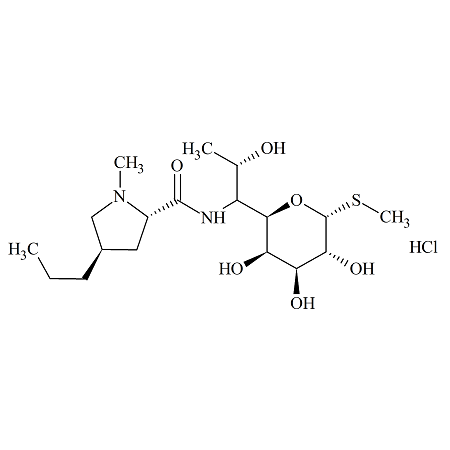
7-Epi-Lincomycin (as Hydrochloride)
- Product Number LIN-15-001
- Parent Drug Lincomycin
- CAS Number 26389-84-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Sorafenib EP Impurity B
- Product Number S-90507-02
- Parent Drug Sorafenib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options