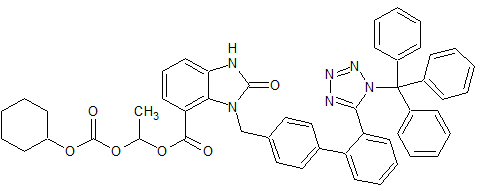
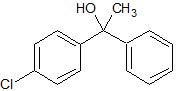
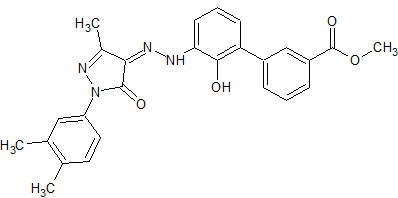
Drug Impurities Reference Standards
Showing 601–610 of 1775 results
-
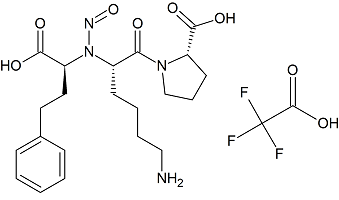
Lisinopril N-Nitroso
- Product Number L-10429-01
- Parent Drug Lisinopril
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Candesartan Cilexetil EP Impurity H
- Product Number CT-01110-561
- Parent Drug Candesartan
- CAS Number 170791-09-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Clemastine EP Impurity C
- Product Number CT-01110-1026
- Parent Drug Clemastine
- CAS Number 59767-24-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Eltrombopag Methyl Ester
- Product Number CT-01110-523
- Parent Drug Eltrombopag
- CAS Number 1246929-01-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Fosfomycin Trometamol EP Impurity C
- Product Number CT-01110-626
- Parent Drug Fosfomycin
- CAS Number 23001-39-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
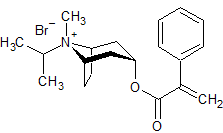
Ipratropium EP Impurity F
- Product Number CT-01110-904
- Parent Drug Ipratropium
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
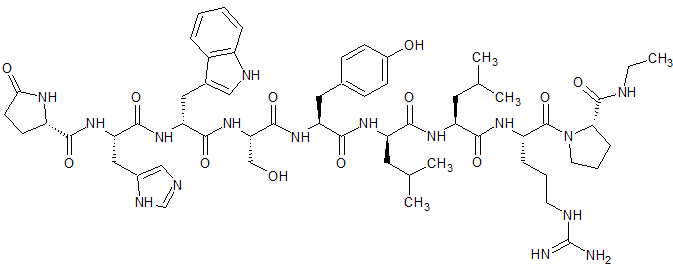
Leuprorelin EP Impurity E
- Product Number CT-01110-1002
- Parent Drug Leuprorelin
- CAS Number 1926163-23-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
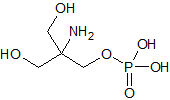
Methyldopa impurity 1
- Product Number CT-01110-937
- Parent Drug Methyldopa
- CAS Number 120-47-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
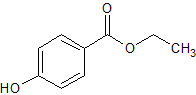
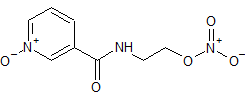
Nicorandil N-Oxide
- Product Number CT-01110-1065
- Parent Drug Nicorandil
- CAS Number 107833-98-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
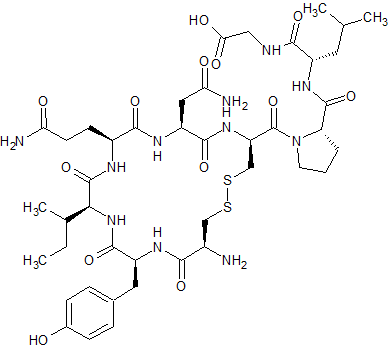
Gly-OH9-Oxytocin
- Product Number CT-01110-1113
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options