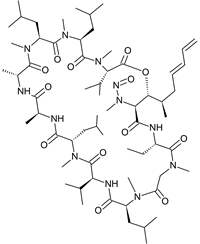Drug Impurities Reference Standards
Showing 701–710 of 1775 results
-
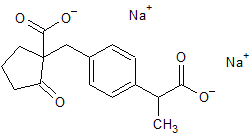
Loxoprofen Impurity 37
- Product Number CT-01110-841
- Parent Drug Loxoprofen
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
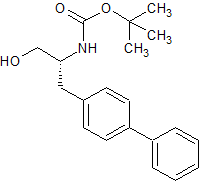
LCZ-696 Intermediate 39
- Product Number CT-01110-947
- Parent Drug LCZ-696
- CAS Number 1426129-50-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
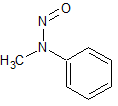
N-nitrosomethylphenylamine (NMPA)
- Product Number N-10521-07
- Parent Drug Nitroso Compounds
- CAS Number 614-00-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
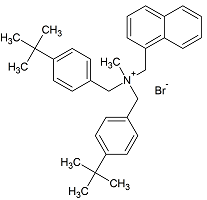
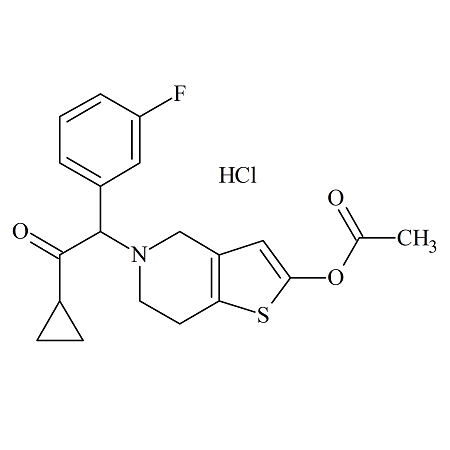
Butenafine Bis(t-butylbenzyl) Bromide
- Product Number B-11029-05
- Parent Drug Butenafine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4-5 week(s)See more size options -
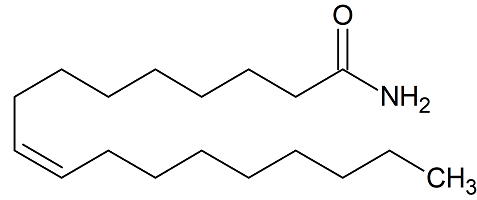
Oleamide Certified Reference Standard
- Product Number FCD-20516-01
- Parent Drug Fine Chemicals
- CAS Number 301-02-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
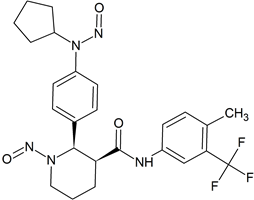
Avacopan Bis-Nitroso Impurity 3
- Product Number A-50716-01
- Parent Drug Avacopan
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Iso Voclosporine N-nitroso
- Product Number V-50124-01
- Parent Drug Voclosporine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
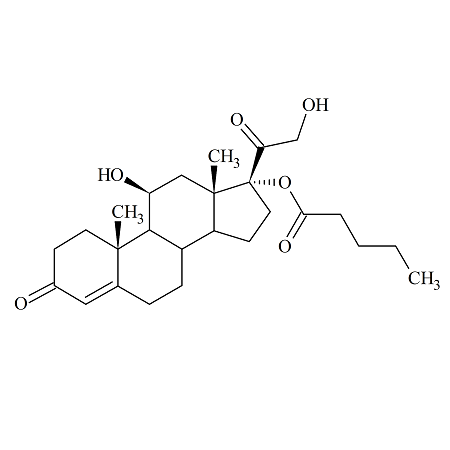
Hydrocortisone 17-Valerate
- Product Number HCO-16-011
- Parent Drug Hydrocortisone
- CAS Number 57524-89-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
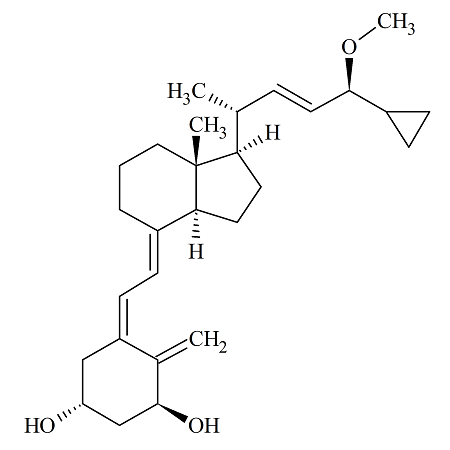
Calcipotriol Methyl Ether
- Product Number ACB-170818-0001
- Parent Drug Calcipotriol
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options