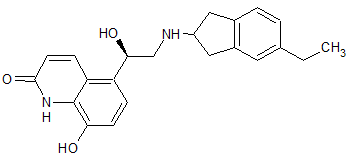
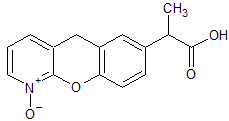
Drug Impurities Reference Standards
Showing 1221–1230 of 1775 results
-
Lansoprazole Impurity 10
- Product Number CT-01110-381
- Parent Drug Lansoprazole
- CAS Number 1083100-27-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
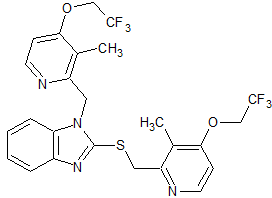
Lenvatinib Impurity 15
- Product Number CT-01110-433
- Parent Drug Lenvatinib
- CAS Number 796848-80-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
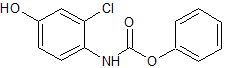
Fosaprepitant Impurity 18
- Product Number CT-01110-490
- Parent Drug Aprepitant
- CAS Number 252742-72-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
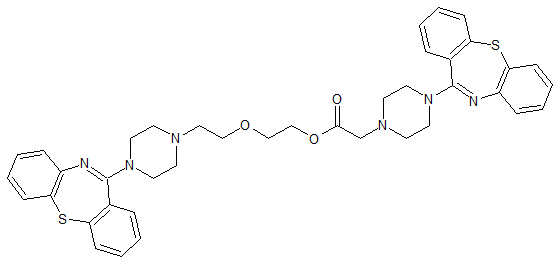
Quetiapine Impurity 18
- Product Number CT-01110-584
- Parent Drug Quetiapine
- CAS Number 1798840-31-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
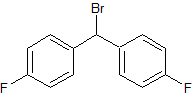
Flunarizine Impurity 16
- Product Number CT-01110-658
- Parent Drug Flunarizine
- CAS Number 345-90-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Glipizide Impurity 14
- Product Number CT-01110-748
- Parent Drug Glipizide
- CAS Number 35303-76-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Indacaterol Impurity 12
- Product Number CT-01110-848
- Parent Drug Indacaterol
- CAS Number 1026461-20-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pranoprofen Impurity 21
- Product Number CT-01110-998
- Parent Drug Pranoprofen
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
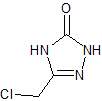
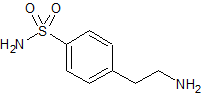
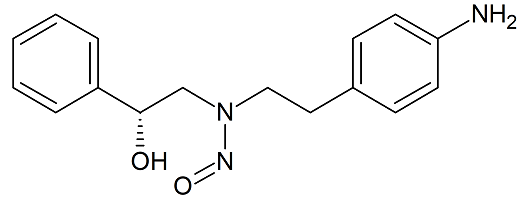
Mirabegron Related Compound 1 N-Nitroso
- Product Number M-10521-01
- Parent Drug Mirabegron
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Imiquimod Aryl 4-Hydroxy
- Product Number I-20106-02
- Parent Drug Imiquimod
- CAS Number 240830-96-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options