Drug Impurities Reference Standards
Showing 151–160 of 1775 results
-
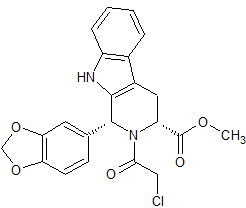
Tadalafil Impurity 37
- Product Number CT-01110-268
- Parent Drug Tadalafil
- CAS Number 171489-59-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ticagrelor Impurity 41
- Product Number CT-01110-316
- Parent Drug Ticagrelor
- CAS Number 376608-65-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Linagliptin Impurity 57
- Product Number CT-01110-364
- Parent Drug Linagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
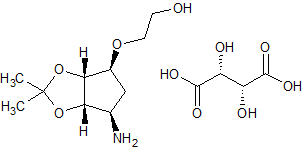
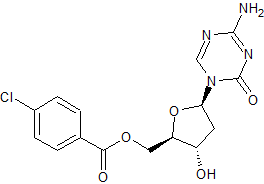
Decitabine Impurity 10
- Product Number CT-01110-425
- Parent Drug Decitabine
- CAS Number 1442660-65-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
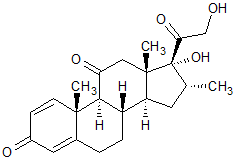
Dexamethasone EP Impurity J
- Product Number CT-01110-478
- Parent Drug Dexamethasone
- CAS Number 2036-77-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Candesartan Cilexetil EP Impurity E
- Product Number CT-01110-559
- Parent Drug Candesartan
- CAS Number 914613-35-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
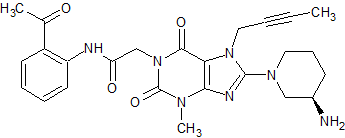
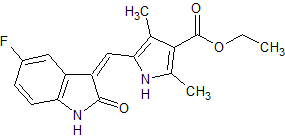
Sunitinib Impurity 27
- Product Number CT-01110-648
- Parent Drug Sunitinib
- CAS Number 1104253-05-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
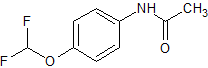
Pantoprazole Impurity 35
- Product Number CT-01110-732
- Parent Drug Pantoprazole
- CAS Number 22236-11-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
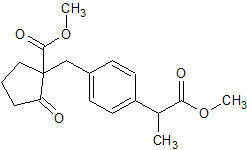
Loxoprofen Impurity 30
- Product Number CT-01110-839
- Parent Drug Loxoprofen
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
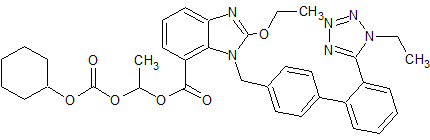
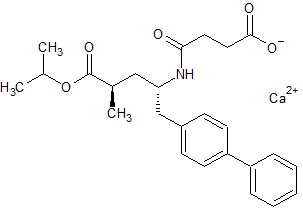
LCZ-696 Impurity 20
- Product Number CT-01110-944
- Parent Drug LCZ-696
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options