Drug Impurities Reference Standards
Showing 171–180 of 1775 results
-
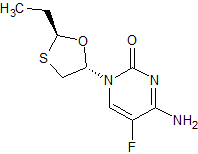
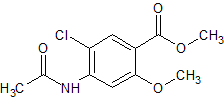
2-epi-Emtricitabine
- Product Number CT-01110-556
- Parent Drug Emtricitabine
- CAS Number 145416-34-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
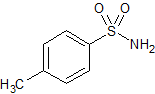
Gliclazide EP Impurity A
- Product Number CT-01110-811
- Parent Drug Gliclazide
- CAS Number 70-55-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
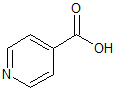
Isoniazid Impurity 2
- Product Number CT-01110-759
- Parent Drug Isoniazid
- CAS Number 55-22-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
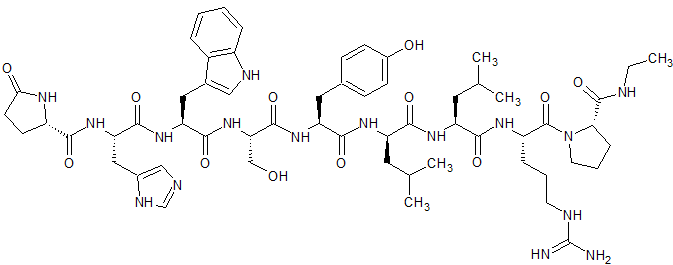
Leuprorelin EP Impurity G
- Product Number CT-01110-1004
- Parent Drug Leuprorelin
- CAS Number 112710-57-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Metoclopramide EP Impurity B
- Product Number CT-01110-771
- Parent Drug Metoclopramide
- CAS Number 4093-31-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Nifedipine Impurity 9
- Product Number CT-01110-766
- Parent Drug Nifedipine
- CAS Number 21829-09-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
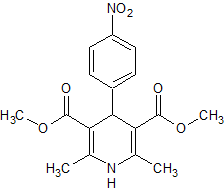
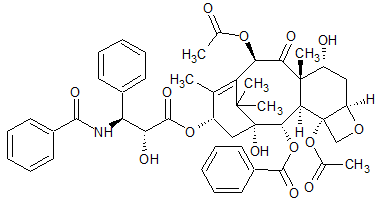
Paclitaxel EP Impurity E
- Product Number CT-01110-1033
- Parent Drug Paclitaxel
- CAS Number 105454-04-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pranoprofen Impurity 8
- Product Number CT-01110-1000
- Parent Drug Pranoprofen
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
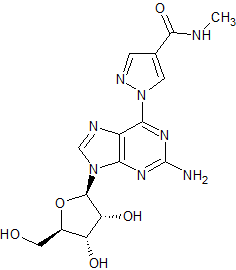
Regadenoson Impurity 4
- Product Number CT-01110-1098
- Parent Drug Regadenoson
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Telmisartan EP Impurity H
- Product Number CT-01110-672
- Parent Drug Telmisartan
- CAS Number 114772-40-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options