Drug Impurities Reference Standards
Showing 181–190 of 1775 results
-
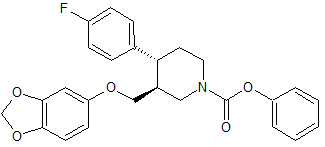
Paroxetine Impurity 30
- Product Number CT-01110-89
- Parent Drug Paroxetine
- CAS Number 253768-88-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
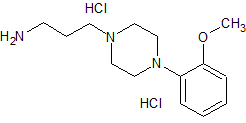
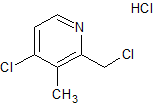
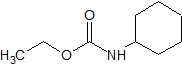
Urapidil Impurity 13
- Product Number CT-01110-1076
- Parent Drug Urapidil
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
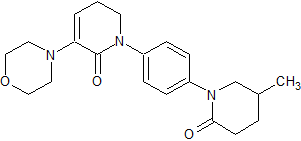
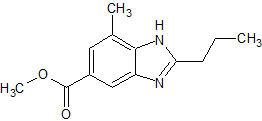
Apixaban Impurity 30
- Product Number CT-01110-280
- Parent Drug Apixaban
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
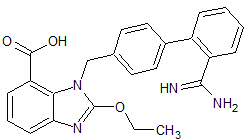
Azilsartan Impurity 15
- Product Number CT-01110-334
- Parent Drug Azilsartan
- CAS Number 1442400-65-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Lansoprazole Impurity 28
- Product Number CT-01110-385
- Parent Drug Lansoprazole
- CAS Number 152402-97-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
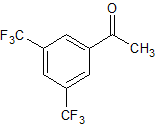
Aprepitant Impurity 34
- Product Number CT-01110-436
- Parent Drug Aprepitant
- CAS Number 30071-93-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
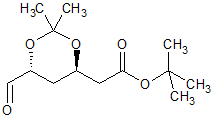
Rosuvastatin Impurity 30
- Product Number CT-01110-493
- Parent Drug Rosuvastatin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
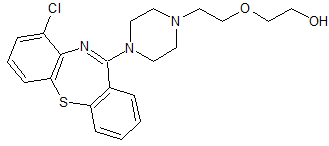
Quetiapine EP Impurity L
- Product Number CT-01110-588
- Parent Drug Quetiapine
- CAS Number 1371638-11-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Telmisartan Impurity 13
- Product Number CT-01110-664
- Parent Drug Telmisartan
- CAS Number 152628-00-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Glipizide Impurity 22
- Product Number CT-01110-752
- Parent Drug Glipizide
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options