Drug Impurities Reference Standards
Showing 1591–1600 of 1775 results
-
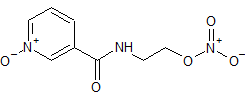
Nicorandil N-Oxide
- Product Number CT-01110-1065
- Parent Drug Nicorandil
- CAS Number 107833-98-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
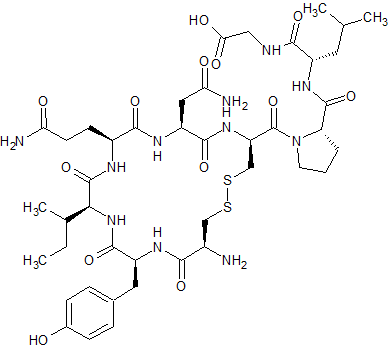
Gly-OH9-Oxytocin
- Product Number CT-01110-1113
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pomalidomide Impurity 7
- Product Number CT-01110-656
- Parent Drug Pomalidomide
- CAS Number 5434-21-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
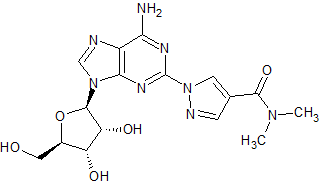
Regadenoson Impurity 2
- Product Number CT-01110-1096
- Parent Drug Regadenoson
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
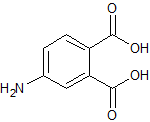
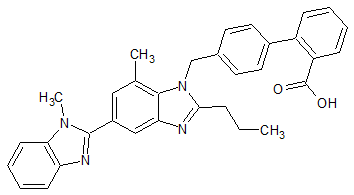
Telmisartan EP Impurity B
- Product Number CT-01110-665
- Parent Drug Telmisartan
- CAS Number 1026353-20-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
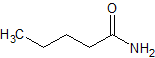
Sodium Valproate Impurity 6
- Product Number CT-01110-487
- Parent Drug Valproic Acid
- CAS Number 626-97-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
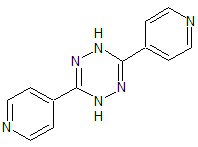
Topiroxostat Impurity 25
- Product Number CT-01110-125
- Parent Drug Topiroxostat
- CAS Number 31599-25-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
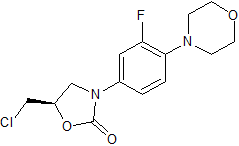
Linezolid Impurity 29
- Product Number CT-01110-172
- Parent Drug Linezolid
- CAS Number 496031-57-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
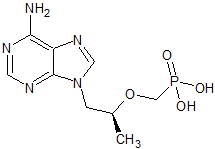
Tenofovir Disoproxil Impurity 26
- Product Number CT-01110-216
- Parent Drug Tenofovir
- CAS Number 147127-19-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 33
- Product Number CT-01110-54
- Parent Drug Vonoprazan
- CAS Number 881674-58-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options