Drug Impurities Reference Standards
Showing 1611–1620 of 1775 results
-
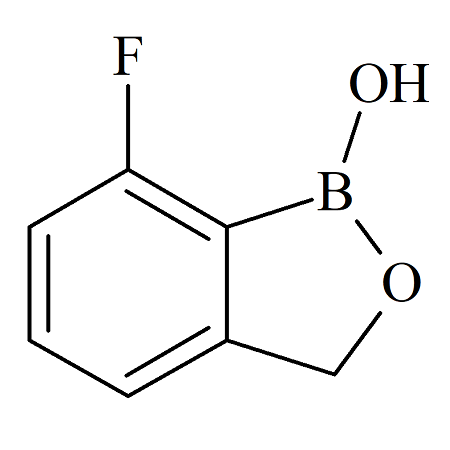
Tavaborole 7-Fluoro Impurity Certified Reference Standard
- Product Number B-70915-0010
- Parent Drug Tavaborole
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
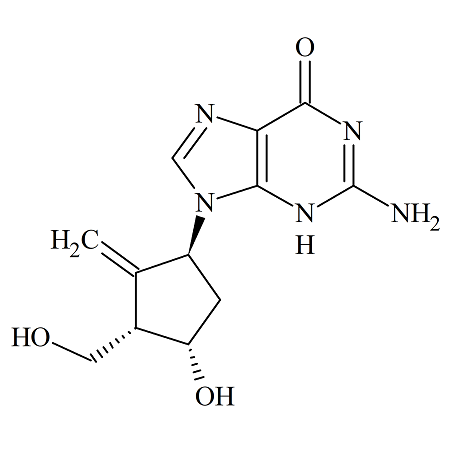
Entecavir (1S,3S,4S) Diastereomer
- Product Number ENT-13-007
- Parent Drug Entecavir
- CAS Number 1367369-77-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
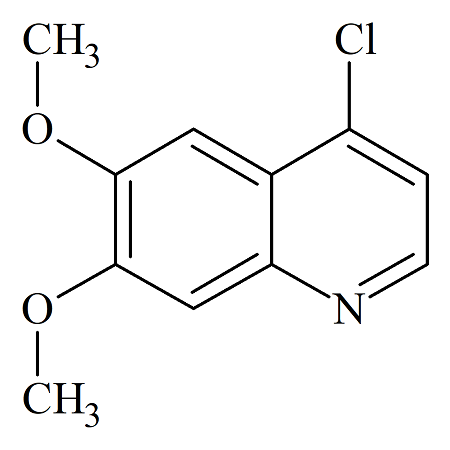
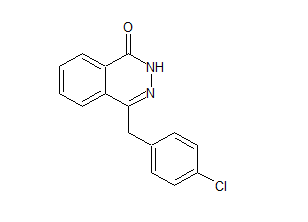
Cabozantinib 4-Chloroquinoline Impurity
- Product Number B-70831-12
- Parent Drug Cabozantinib
- CAS Number 35654-56-9
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
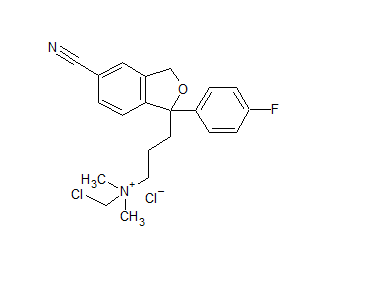
Citalopram Chloromethyl Quarternary Ammonium Salt Impurity
- Product Number CIT-20-001
- Parent Drug Citalopram
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
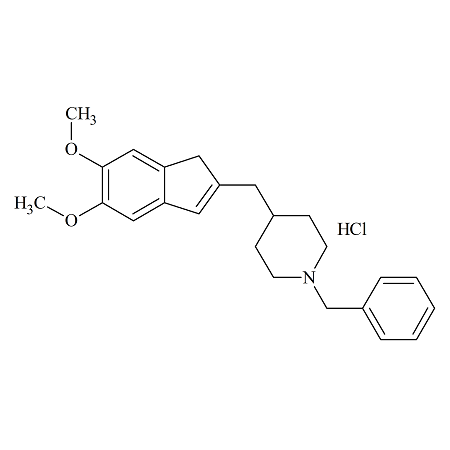
Dehydrodeoxy Donepezil
- Product Number DON-10-004
- Parent Drug Donepezil
- CAS Number 120013-45-8
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
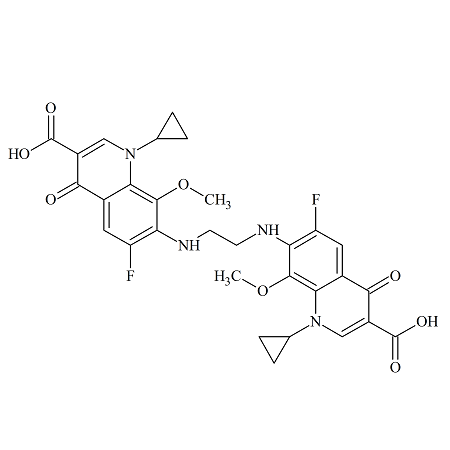
Gatifloxacin Dimer 4
- Product Number ACA-161006-0001
- Parent Drug Gatifloxacin
- CAS Number 1497338-53-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
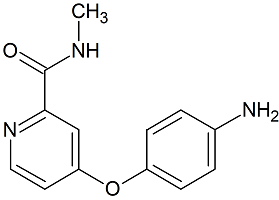
Sorafenib EP Impurity A
- Product Number S-90507-01
- Parent Drug Sorafenib
- CAS Number 284462-37-9
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
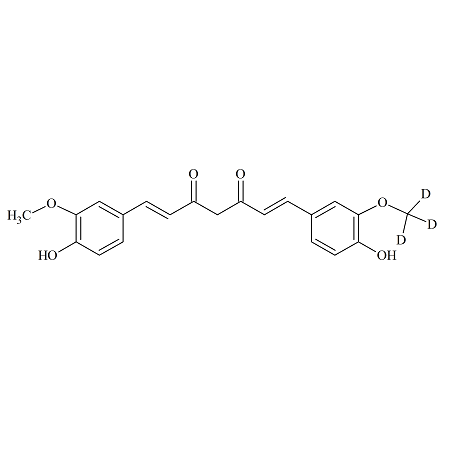
Curcumin-D3
- Product Number CUR-16-003
- Parent Drug Curcumin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 1 week(s)See more size options