Drug Impurities Reference Standards
Showing 1601–1610 of 1775 results
-
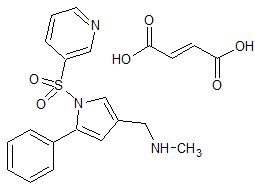
Vonoprazan Fumarate Impurity 45
- Product Number CT-01110-61
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
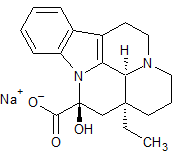
Vinpocetine Impurity 12
- Product Number CT-01110-1036
- Parent Drug Vinpocetine
- CAS Number 59413-21-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
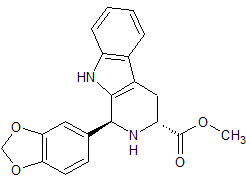
Tadalafil Impurity 15
- Product Number CT-01110-258
- Parent Drug Tadalafil
- CAS Number 171596-42-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
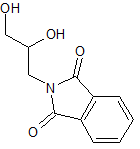
Rivaroxaban Impurity 48
- Product Number CT-01110-303
- Parent Drug Rivaroxaban
- CAS Number 62457-35-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
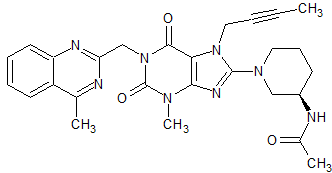
Linagliptin Impurity 38
- Product Number CT-01110-356
- Parent Drug Linagliptin
- CAS Number 1803079-49-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
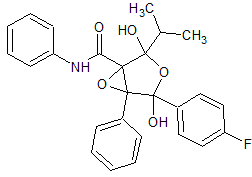
Atorvastatin Epoxy Tetrahydrofuran Impurity
- Product Number CT-01110-415
- Parent Drug Atorvastatin
- CAS Number 873950-19-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
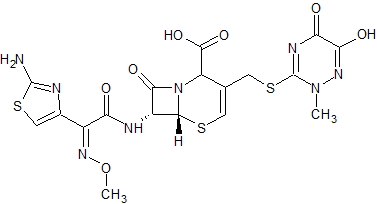
Ceftriaxone Impurity 14
- Product Number CT-01110-466
- Parent Drug Ceftriaxone
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
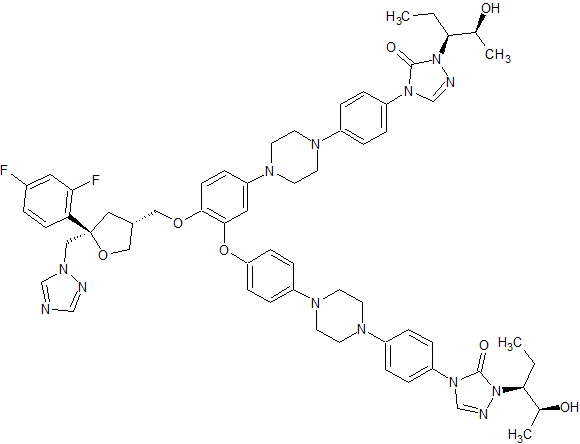
Posaconazole Impurity 62
- Product Number CT-01110-538
- Parent Drug Posaconazole
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Mesalazine EP Impurity M
- Product Number CT-01110-631
- Parent Drug Mesalamine
- CAS Number 2516-96-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Levofloxacin 9-Piperazinyl Isomer
- Product Number CT-01110-701
- Parent Drug Levofloxacin
- CAS Number 178912-62-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options