Drug Impurities Reference Standards
Showing 1621–1630 of 1775 results
-
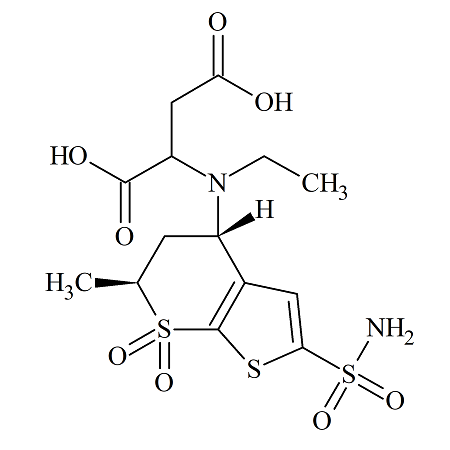
Dorzolamide Maleic Acid Addition Impurity (Mixture of Diastereomers)
- Product Number DOR-14-002
- Parent Drug Dorzolamide
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
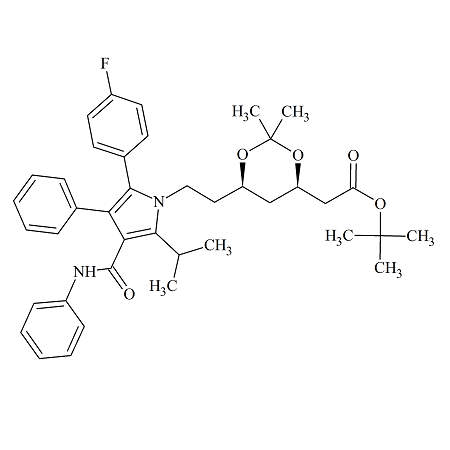
Fingolimod Impurity 32
- Product Number F-90917-02
- Parent Drug Fingolimod
- CAS Number 851039-24-2
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
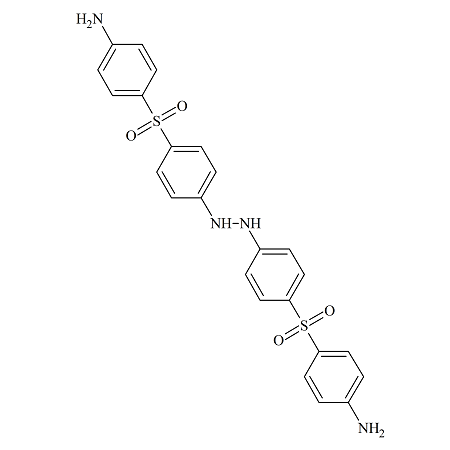
Dapsone Hydrazine Dimer
- Product Number DAP-15-004
- Parent Drug Dapsone
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
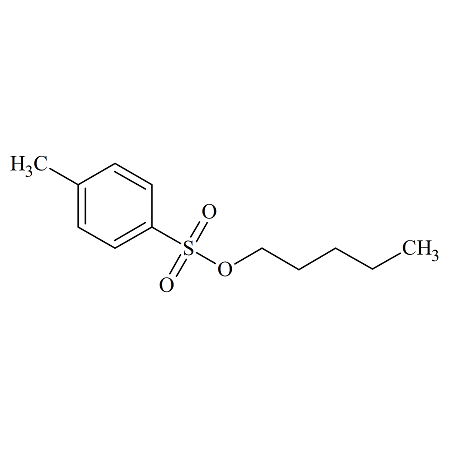
n-Pentyl Toluenesulfonate
- Product Number TOS-12-002
- Parent Drug Toluenesulfonates
- CAS Number 4450-76-4
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
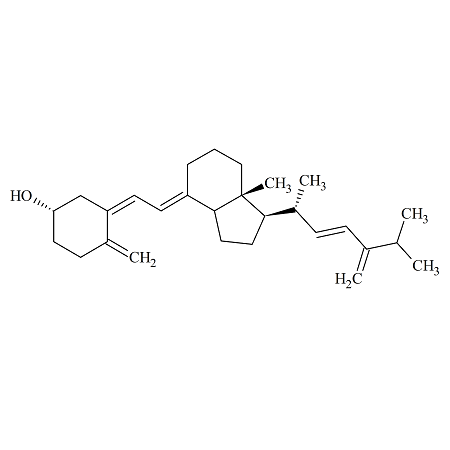
Ergocalciferol Dehydro Impurity
- Product Number ERG-09-001
- Parent Drug Vitamin D2
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
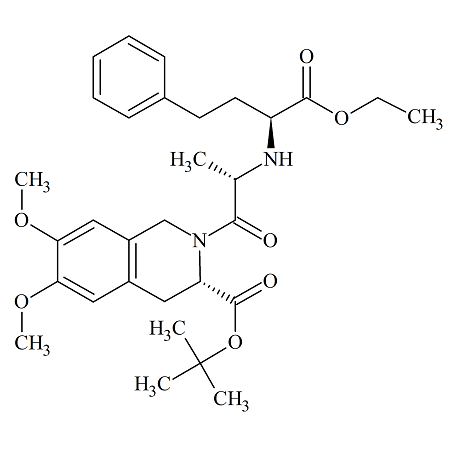
Moexipril USP RC C
- Product Number MOE-12-004
- Parent Drug Moexipril
- CAS Number 103733-39-7
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
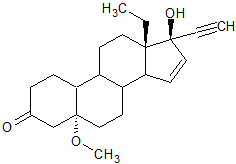
Gestodene Impurity I
- Product Number G-90916-02
- Parent Drug Gestodene
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
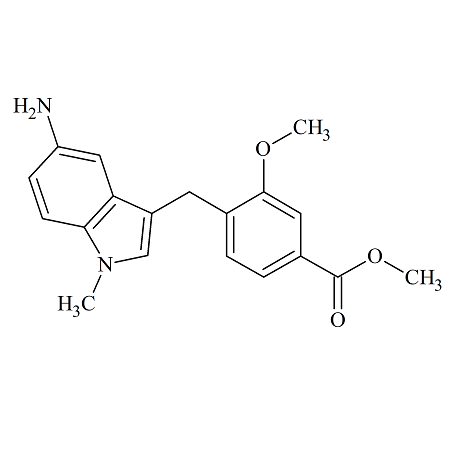
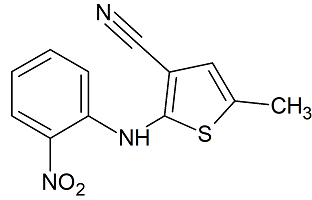
Zafirlukast 5-Aminoindole Impurity
- Product Number ACB-170824-0004
- Parent Drug Zafirlukast
- CAS Number 107754-14-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Olanzapine Related Compound A
- Product Number O-10208-01
- Parent Drug Olanzapine
- CAS Number 138564-59-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options