Drug Impurities Reference Standards
Showing 1681–1690 of 1775 results
-
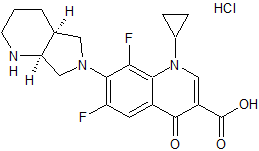
Moxifloxacin EP Impurity A
- Product Number CT-01110-181
- Parent Drug Moxifloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
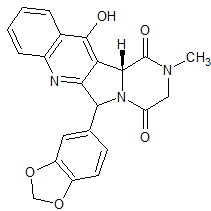
Tadalafil EP Impurity G
- Product Number CT-01110-275
- Parent Drug Tadalafil
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Olmesartan Impurity 3
- Product Number CT-01110-343
- Parent Drug Olmesartan
- CAS Number 80841-78-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Rabeprazole Impurity 9
- Product Number CT-01110-409
- Parent Drug Rabeprazole
- CAS Number 1114543-47-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 5
- Product Number CT-01110-65
- Parent Drug Vonoprazan
- CAS Number 881677-11-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Bifonazole EP Impurity C(Clotrimazole EP Impurity D)
- Product Number CT-01110-727
- Parent Drug Bifonazole
- CAS Number 288-32-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Cefpodoxime Proxetil EP Impurity E
- Product Number CT-01110-878
- Parent Drug Cefpodoxime
- CAS Number 217803-89-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Doxofylline Impurity 2
- Product Number CT-01110-863
- Parent Drug Doxofylline
- CAS Number 5614-53-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Flumazenil EP Impurity A
- Product Number CT-01110-785
- Parent Drug Flumazenil
- CAS Number 84378-44-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Hydroxychloroquine EP Impurity G
- Product Number CT-01110-1025
- Parent Drug Hydroxychloroquine
- CAS Number 86-98-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options