Drug Impurities Reference Standards
Showing 1691–1700 of 1775 results
-
Glipizide EP Impurity I
- Product Number CT-01110-758
- Parent Drug Glipizide
- CAS Number 10079-35-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
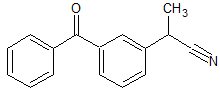
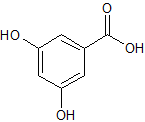
Ketoprofen EP Impurity F
- Product Number CT-01110-826
- Parent Drug Ketoprofen
- CAS Number 42872-30-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
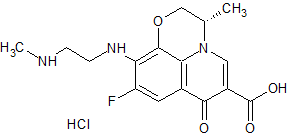
Levofloxacin Diamine Derivative
- Product Number CT-01110-709
- Parent Drug Levofloxacin
- CAS Number 1346603-62-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
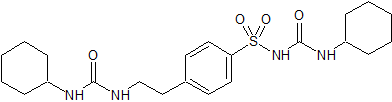
Metoprolol EP Impurity D
- Product Number CT-01110-692
- Parent Drug Metoprolol
- CAS Number 62572-90-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
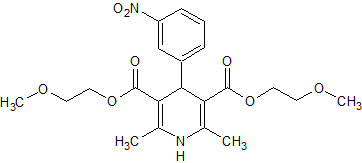
Nimodipine EP Impurity C
- Product Number CT-01110-717
- Parent Drug Nimodipine
- CAS Number 70172-96-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
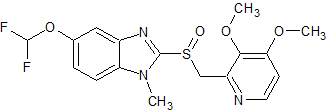
Pantoprazole EP Impurity D
- Product Number CT-01110-734
- Parent Drug Pantoprazole
- CAS Number 624742-53-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Prilocaine EP Impurity F
- Product Number CT-01110-1085
- Parent Drug Prilocaine
- CAS Number 13327-14-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
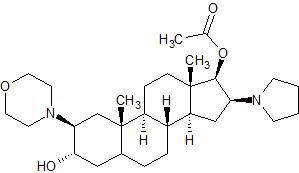
Rocuronium EP Impurity A
- Product Number CT-01110-576
- Parent Drug Rocuronium
- CAS Number 119302-24-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Terbutaline EP Impurity A
- Product Number CT-01110-509
- Parent Drug Terbutaline
- CAS Number 36438
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
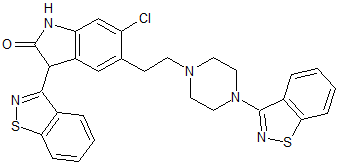
Ziprasidone EP Impurity E
- Product Number CT-01110-573
- Parent Drug Ziprasidone
- CAS Number 1159977-04-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options