Drug Impurities Reference Standards
Showing 1661–1670 of 1775 results
-
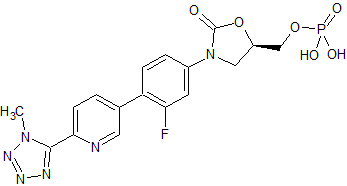
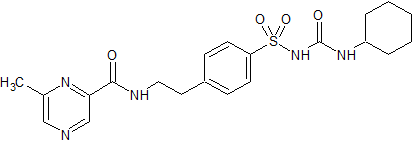
Tedizolid Impurity 7
- Product Number CT-01110-124
- Parent Drug Tedizolid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
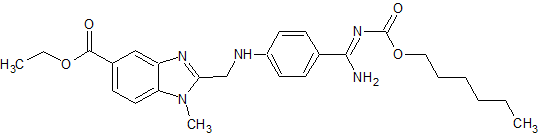
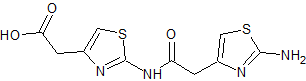
Moxifloxacin impurity 9
- Product Number CT-01110-208
- Parent Drug Moxifloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Dabigatran Impurity 4
- Product Number CT-01110-290
- Parent Drug Dabigatran
- CAS Number 1408238-36-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
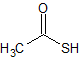
Racecadotril EP Impurity A
- Product Number CT-01110-366
- Parent Drug Racecadotril
- CAS Number 507-09-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
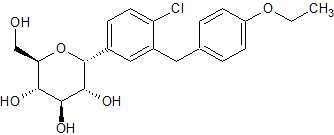
Dapagliflozin Impurity 1
- Product Number CT-01110-426
- Parent Drug Dapagliflozin
- CAS Number 1373321-04-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Mirabegron Impurity 7
- Product Number CT-01110-86
- Parent Drug Mirabegron
- CAS Number 2036283-13-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
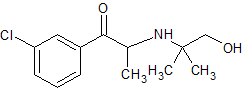
Bupropion Impurity 7
- Product Number CT-01110-536
- Parent Drug Bupropion
- CAS Number 92264-81-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
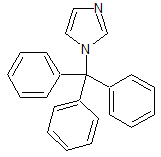
Clotrimazole EP Impurity F
- Product Number CT-01110-871
- Parent Drug Clotrimazole
- CAS Number 15469-97-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
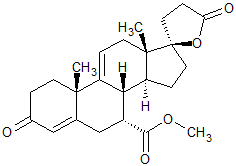
Eplerenone EP Impurity C
- Product Number CT-01110-898
- Parent Drug Eplerenone
- CAS Number 95716-70-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Glipizide EP Impurity E
- Product Number CT-01110-756
- Parent Drug Glipizide
- CAS Number 66375-96-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options