Drug Impurities Reference Standards
Showing 191–200 of 1775 results
-
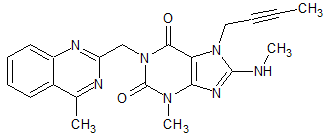
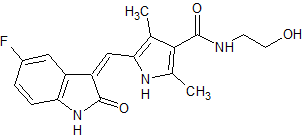
Linagliptin Impurity 47
- Product Number CT-01110-360
- Parent Drug Linagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
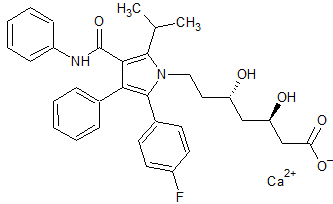
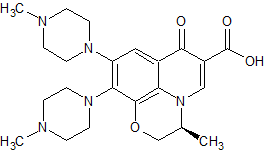
Atorvastatin Impurity 51
- Product Number CT-01110-419
- Parent Drug Atorvastatin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
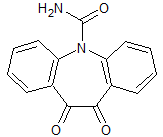
Oxcarbazepine EP Impurity I
- Product Number CT-01110-474
- Parent Drug Oxcarbazepine
- CAS Number 537693-29-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
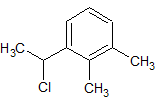
Dexmedetomidine Impurity 16
- Product Number CT-01110-551
- Parent Drug Dexmedetomidine
- CAS Number 60907-88-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Sunitinib Impurity 21
- Product Number CT-01110-644
- Parent Drug Sunitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
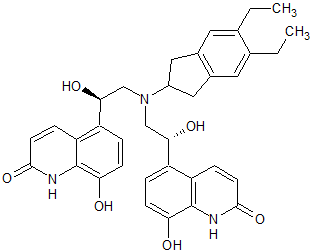
Levofloxacin Impurity 32
- Product Number CT-01110-707
- Parent Drug Levofloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Indacaterol Impurity 16
- Product Number CT-01110-850
- Parent Drug Indacaterol
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
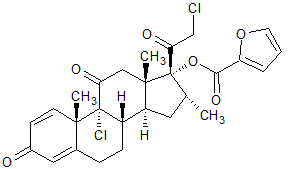
Mometasone Furoate Impurity 11
- Product Number CT-01110-953
- Parent Drug Mometasone
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Rasagiline Mesylate Impurity B
- Product Number I-30905-01
- Parent Drug Rasagiline Mesylate
- CAS Number 10277-74-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
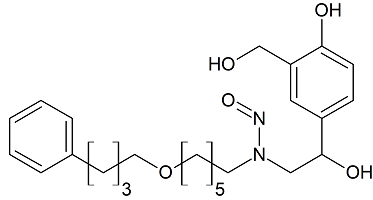
Salmeterol N-nitroso
- Product Number S-30914-01
- Parent Drug Salmeterol
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options