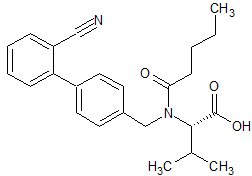
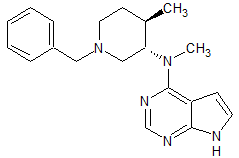
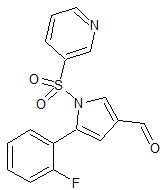
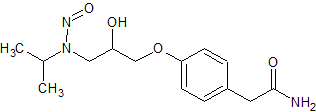
Drug Impurities Reference Standards
Showing 611–620 of 1775 results
-
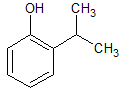
Propofol EP Impurity C
- Product Number CT-01110-828
- Parent Drug Propofol
- CAS Number 88-69-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
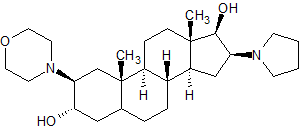
Rocuronium EP Impurity G
- Product Number CT-01110-580
- Parent Drug Rocuronium
- CAS Number 119302-20-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
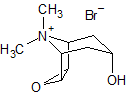
Tiotropium EP Impurity G
- Product Number CT-01110-977
- Parent Drug Tiotropium
- CAS Number 1508-46-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
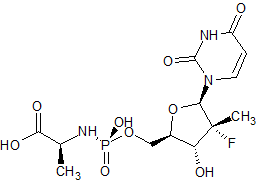
Sofosbuvir Impurity 51
- Product Number CT-01110-108
- Parent Drug Sofosbuvir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Valsartan Impurity 42
- Product Number CT-01110-138
- Parent Drug Valsartan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Moxifloxacin Impurity 29
- Product Number CT-01110-191
- Parent Drug Moxifloxacin
- CAS Number 1395056-41-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 39
- Product Number CT-01110-236
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 64
- Product Number CT-01110-68
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Atenolol N-Nitroso
- Product Number A-10521-03
- Parent Drug Atenolol
- CAS Number 134720-04-0
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
Tadalafil Impurity 31
- Product Number CT-01110-266
- Parent Drug Tadalafil
- CAS Number 2058231-08-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options