Drug Impurities Reference Standards
Showing 641–650 of 1775 results
-
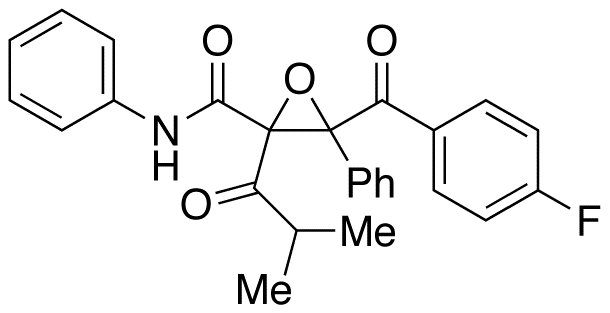
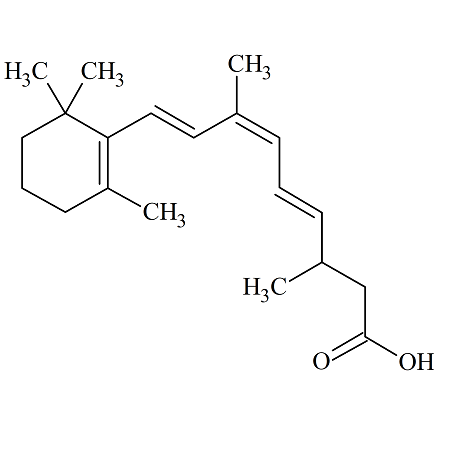
9-Cis-13,14-Dihydro Retinoic Acid
- Product Number ACA-160929-0001
- Parent Drug Retinoic Acid
- CAS Number 176019-01-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
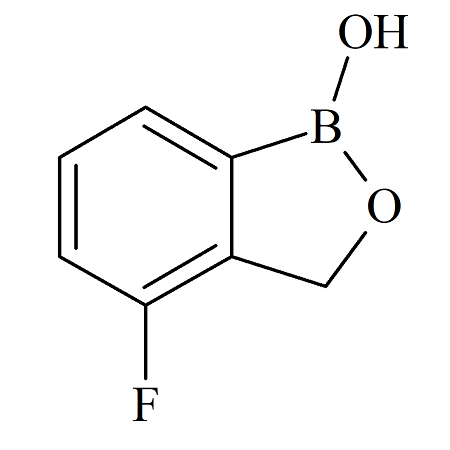
Tavaborole 4-Fluoro Impurity Certified Reference Standard
- Product Number B-70915-0008
- Parent Drug Tavaborole
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
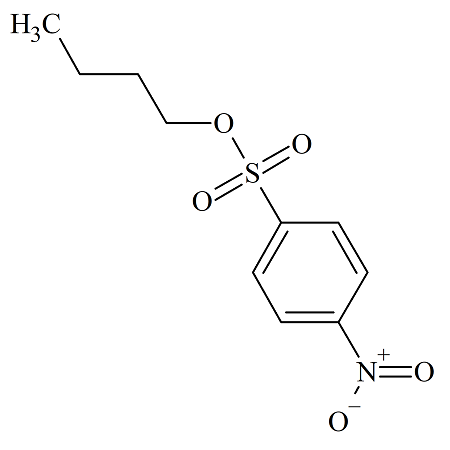
n-Butyl 4-Nitrobenzenesulfonate
- Product Number NBS-10-005
- Parent Drug p-Nitrobenzenesulfonates
- CAS Number 4028-52-8
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
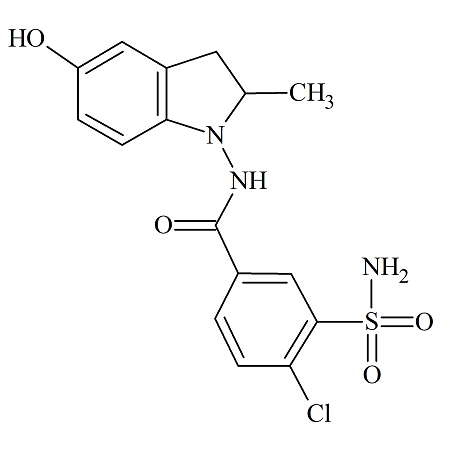
5-Hydroxy Indapamide
- Product Number IND-16-001
- Parent Drug Indapamide
- CAS Number 126750-70-7
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 1 week(s)See more size options -
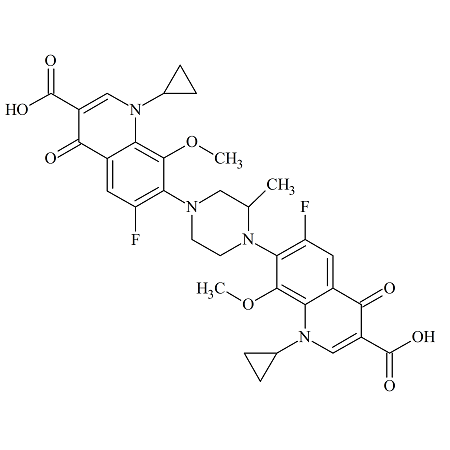
Gatifloxacin Dimer 1
- Product Number ACA-161006-0002
- Parent Drug Gatifloxacin
- CAS Number 1497338-46-1
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
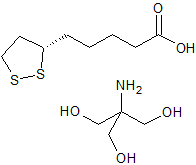
(S)-a-Lipoic Acid Tromethamine Salt
- Product Number L-91101-1
- Parent Drug Lipoic Acid
- CAS Number 248914-06-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
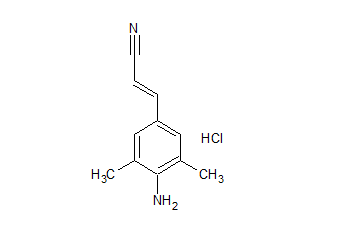
Rilpivirine Dimethylanilinoacrylonitrile Certified Impurity Reference Standard
- Product Number R-00213-05
- Parent Drug Rilpivirine
- CAS Number 661489-23-2
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
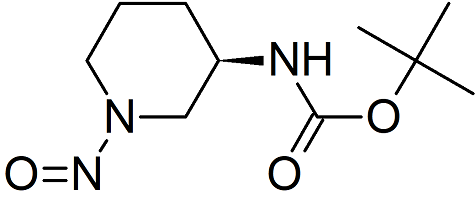
Linagliptin Impurity 4 N-Nitroso
- Product Number L-30405-01
- Parent Drug Linagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options