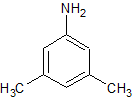
Drug Impurities Reference Standards
Showing 671–680 of 1775 results
-
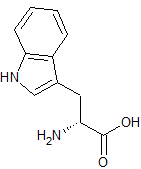
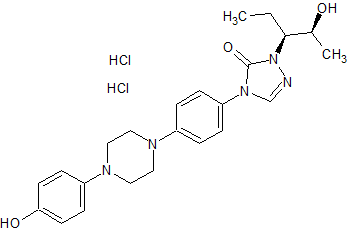
Tadalafil Impurity 18
- Product Number CT-01110-259
- Parent Drug Tadalafil
- CAS Number 153-94-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Rivaroxaban Impurity 82
- Product Number CT-01110-308
- Parent Drug Rivaroxaban
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Linagliptin Impurity 40
- Product Number CT-01110-357
- Parent Drug Linagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
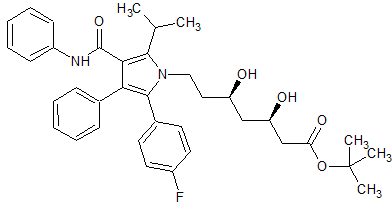
Atorvastatin Impurity 21
- Product Number CT-01110-416
- Parent Drug Atorvastatin
- CAS Number 134395-00-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ceftriaxone Impurity 19
- Product Number CT-01110-467
- Parent Drug Ceftriaxone
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
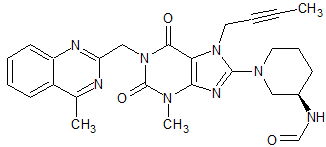
Posaconazole Impurity 66
- Product Number CT-01110-539
- Parent Drug Posaconazole
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
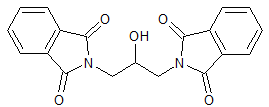
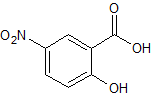
Mesalazine EP Impurity N
- Product Number CT-01110-632
- Parent Drug Mesalamine
- CAS Number 96-97-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
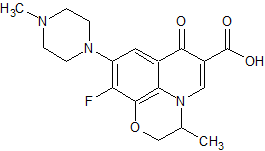
Levofloxacin Impurity 13
- Product Number CT-01110-702
- Parent Drug Levofloxacin
- CAS Number 197291-75-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
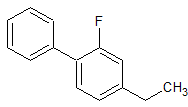
Flurbiprofen Impurity 12
- Product Number CT-01110-805
- Parent Drug Flurbiprofen
- CAS Number 55258-76-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Lidocaine Impurity 19
- Product Number CT-01110-924
- Parent Drug Lidocaine
- CAS Number 108-69-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options