Drug Impurities Reference Standards
Showing 681–690 of 1775 results
-
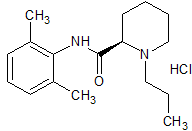
Ropivacaine EP Impurity G
- Product Number CT-01110-974
- Parent Drug Ropivacaine
- CAS Number 112773-90-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
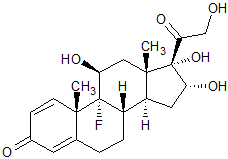
Triamcinolone Acetonide EP Impurity A
- Product Number CT-01110-635
- Parent Drug Triamcinolone
- CAS Number 124-94-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
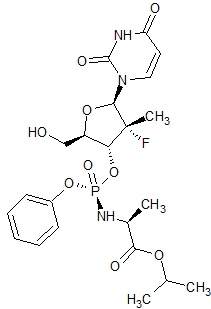
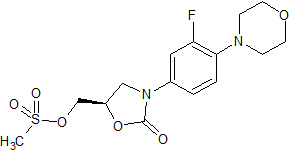
Sofosbuvir Impurity 49
- Product Number CT-01110-107
- Parent Drug Sofosbuvir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
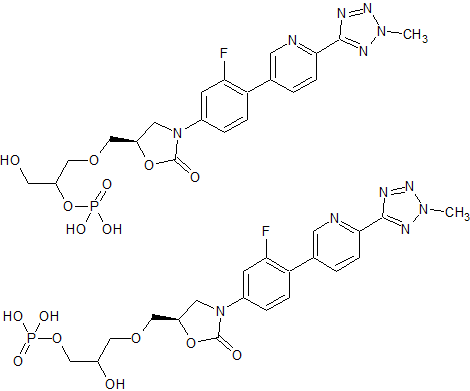
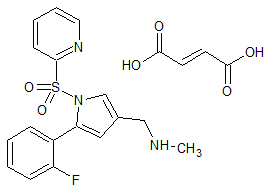
Tedizolid Impurity 40
- Product Number CT-01110-122
- Parent Drug Tedizolid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Linezolid Impurity 22
- Product Number CT-01110-170
- Parent Drug Linezolid
- CAS Number 174649-09-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
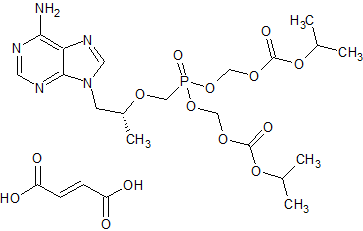
Tenofovir disoproxil Impurity 17
- Product Number CT-01110-213
- Parent Drug Tenofovir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
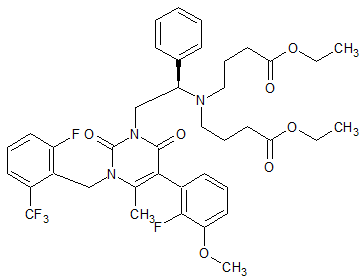
Vonoprazan Fumarate Impurity 23
- Product Number CT-01110-51
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
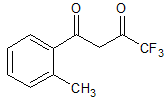
Celecoxib Impurity 22
- Product Number CT-01110-99
- Parent Drug Celecoxib
- CAS Number 163266-02-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Elagolix Impurity 10
- Product Number CT-01110-1116
- Parent Drug Elagolix
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
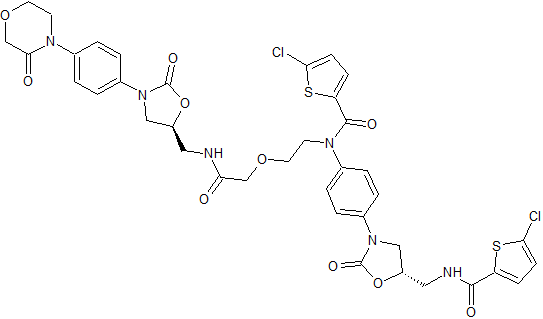
Rivaroxaban Impurity 11
- Product Number CT-01110-293
- Parent Drug Rivaroxaban
- CAS Number 1632463-24-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options